Team:TU Delft/Project/sensing/parts
From 2010.igem.org

Sensing Parts
Introduction
Catabolite repression control
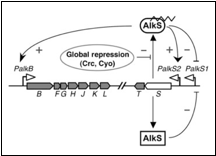
In Pseudomonas putida, the AlkS transcriptional regulator activates the expression of its own gene, and that of alkT, from a promoter named PalkS2 in the presence of alkanes. This allows achieving AlkS levels that are high enough to activate the expression of the alkBFGHJKL operon from the PalkB promoter. AlkS recognizes C5–C10 n-alkanes as effectors, but does not respond to shorter or larger alkanes (Rojo et al. 2009) (figure 1)
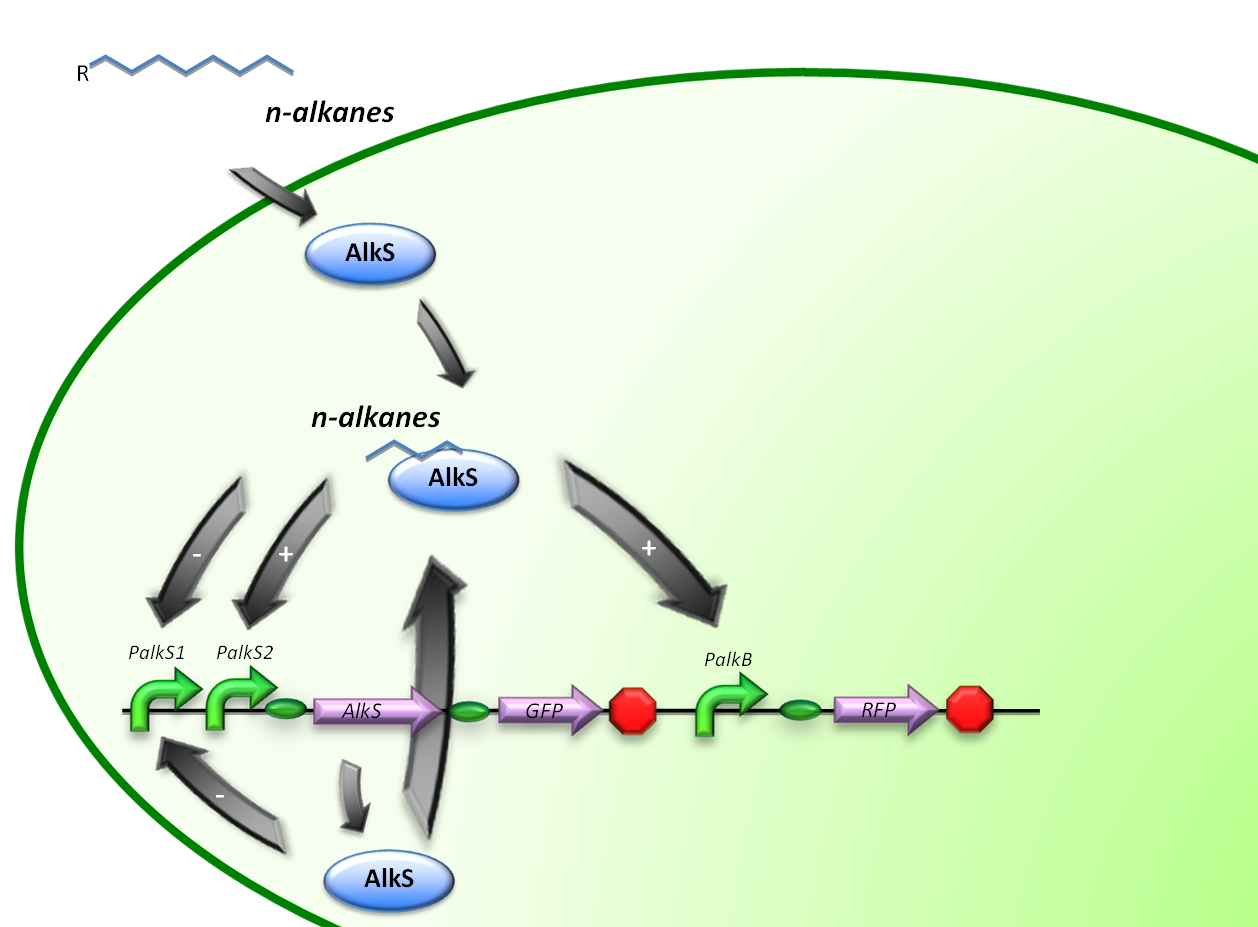
In stead of using all the alkBFGHJKL genes, a GFP will be introduced into our system to check the functionality of the PalkB promoter in E.coli. (figure 2)
Therefore, the first construct is suitable for the analysis of transcriptional activity over the PalkS promoter. A plasmid containing the AlkS-PalkS-PalkB regulatory mechanism will be coupled to GFP and RFP generators in order to determine transcriptional activities of PalkS and PalkB at varying hydrocarbon concentrations by measuring fluorescence.
Control by Crp
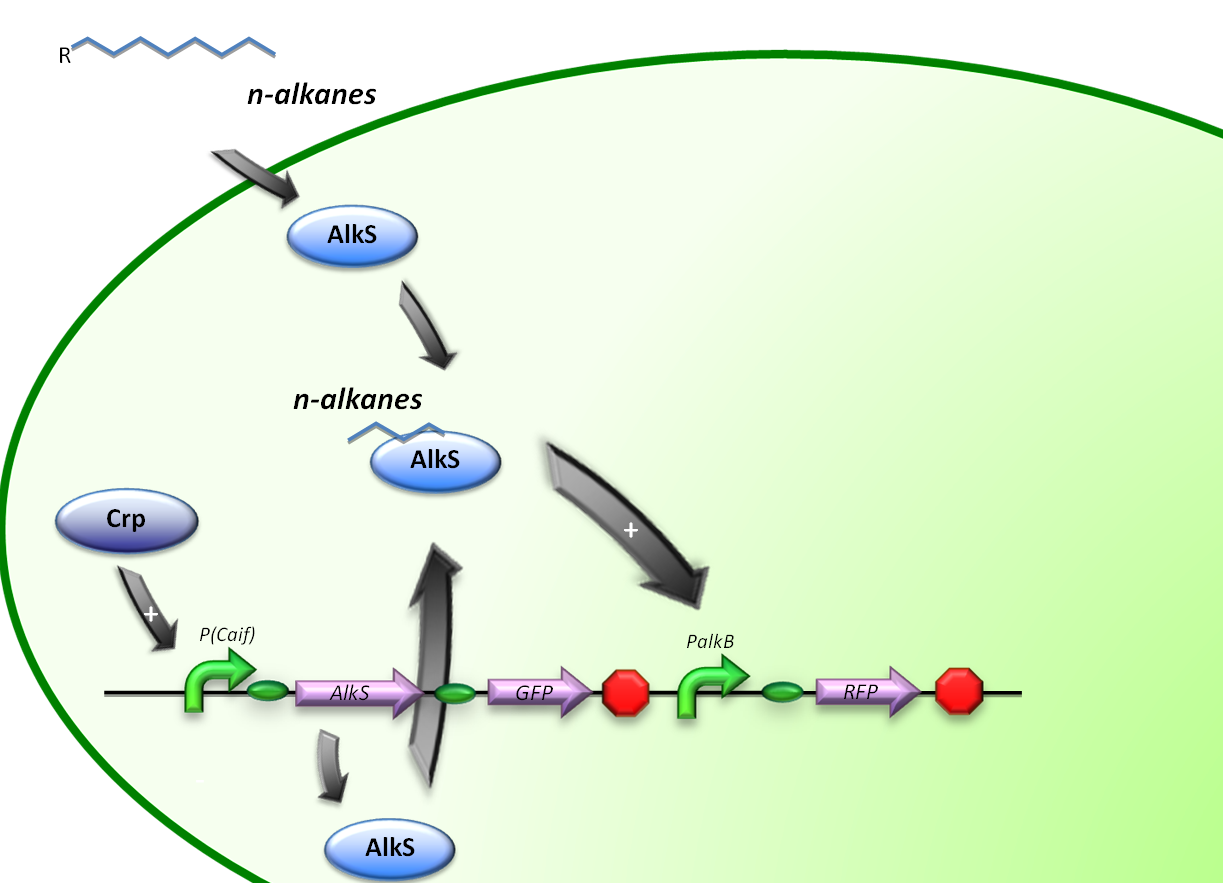
The expression of alkane degrading genes in our second E.coli regulatory system will be regulated by making the alkane-degrading genes sensitive to Crp. The global regulator protein Crp binds to regions of promoters known to activate genes involved in the degradation of non-glucose carbon sources, in this way activating the genes downstream of it.
For the induction of alkane-degradation genes in this construct the BioBrick consisted out of the P(CaiF) promoter, AlkS gene and PalkB gene. (figure 3)
BBa_K398331: P(Caif)-RBS-GFP-TT
pCaiF is a natural promoter found in E. coli K12, pCaiF is part of the translational unit of the protein CaiF which is a transcriptional regulator of carnitine metabolism under anaerobiosis and glucose limitation. From the original sequence, we took the cAMP-Crp and RNApol sigma 70 binding sites. Under low glucose levels, the cellular cAMP (cyclic Adenosine Mononucleotide Phosphate) levels are high and thus Crp (Catabolite gene activator protein) will bind easier to this molecule forming the complex cAMP-Crp. This complex acts as a transcriptional regulator of over 180 proteins involved in metabolism of secondary carbon sources.
The original purpose of pCaiF for our project was to serve as a promotor for our proteins when glucose or other carbon sources easier to degrade are not in the medium, in that way cells will grow faster and once a certain cell density is reached they can start to degrade other carbon sources like alkanes.
Because of the limited amount of time, we just succeeded on the construction of a measurement device in order to characterize pCaiF. An natural occurring E. coli promotor. We hope that other teams will use these sequence in the future for several reasons, among them the necessity of well characterized promoters in the catalog.
View this part in the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K398331 part registry]
References
- Canosa, I., J. M. Sanchez-Romero, et al. A positive feedback mechanism controls expression of AlkS, the transcriptional regulator of the Pseudomonas oleovorans alkane degradation pathway. Molecular Microbiology 35(4): 791-799 (2000)
- Moreno, R., A. Ruiz-Manzano, et al. The Pseudomonas putida Crc global regulator is an RNA binding protein that inhibits translation of the AlkS transcriptional regulator. Molecular Microbiology 64(3): 665-675 (2007)
- van Beilen, J. B., S. Panke, et al. Analysis of Pseudomonas putida alkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk genes. Microbiology-Sgm 147: 1621-1630 (2001)
- Rojo, F. , Degradation of alkanes by bacteria. Environmental Microbiology 11: 2477-2490 (2009)
- Kotte, O, Zaugg, J., Heinemann, M. , ‘Bacterial adaptation through distributed sensing of metabolic fluxes’, Molecular Systems Biology, 6:355, doi:10.1038/msb.2010.10 (2010)
- Kremling, A., Bettenbrock, K., Gilles, E.D., ‘Analysis of global control of Escherichia coli carbohydrate uptake’, BMC Systems Biology, 1:42, doi:10.1186/1752-0509-1-42 (2007)
- Lin, H. Y., Mathiszik, B., Xu, B., Enfors, S.-O., Neubauer, P., ‘Determination of the Maximum Specific Uptake Capacities for Glucose and Oxygen in Glucose-Limited Fed-Batch Cultivations of Escherichia coli’, Biotechnology and Bioengineering, 73, 347-357 (2001)
- Alon, U. (ed.), An Introduction to Systems Biology: Design Principles of Biological Circuits, CRC Press (2007)
- EcoCyc: [http://BioCyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=CPLX0-226 click here]

 "
"