Team:TU Delft/Modeling/MFA/results
From 2010.igem.org
Lbergwerff (Talk | contribs) (→PHB production) |
Lbergwerff (Talk | contribs) (→isoprene production) |
||
| Line 41: | Line 41: | ||
=isoprene production= | =isoprene production= | ||
| - | [[Image:Team_TUDelft_isoprene_data.png|thumb|800px|centre|'''Figure 6''' – | + | Isoprene production was chosen as a hypothetical pathway. Although it is an unlikely product for ''E. coli'', in theory it holds much promise as it is very reduced. The calculated yields from the metabolic flux analysis are displayed in figure 6. |
| + | |||
| + | [[Image:Team_TUDelft_isoprene_data.png|thumb|800px|centre|'''Figure 6''' – The most important yields from the MFA for alkane degradation with production of PHB]] | ||
| + | |||
| + | The product yields for isoprene are lower than for PHB in the previous case. The upper limit is only 0.833 Cmol isoprene per Cmol substrate, as in the first step of the isoprene pathway 2 C3 molecules are used to form a C5 molecule and CO<sub>2</sub>. The yields for isoprene on alkanes are further lowered because the acetyl-CoA (C2) needs to be converted to C3 molecules. This leads to CO<sub>2</sub> production through the anapleorotic routes and therefore in a drop of the yield. This also why short-chained odd numbered alkanes have a higher yield as the ration propionyl-CoA/acetyl-CoA is higher for shorter odd numbered alkanes. The yield for glucose is lower than the absolute maximum because substrate needs to be consumed for production of cofactors that are required for the isoprene pathway. This is also relfected by the fact that no ATP is going to maintenance with glucose as substrate. | ||
=hydrogen production= | =hydrogen production= | ||
Revision as of 21:30, 24 October 2010
Contents |
MFA results
Alkane degradation
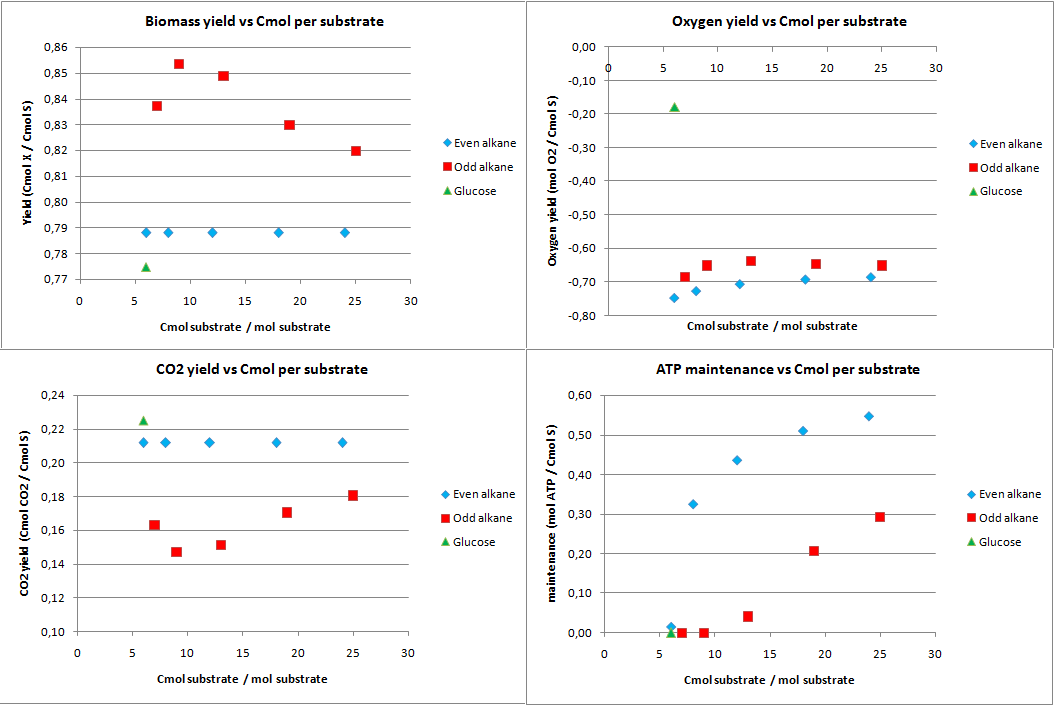
In figure 1 the biomass, oxygen, CO2 yields are plotted against the amount of Cmol of the product. Also the amount of ATP going into maintenance (essentially getting rid of the ATP) is plotted in figure 1. Glucose has 6 carbon atoms and for the alkanes a range 6 carbon atoms to 25 carbon atoms was taken. These results are for the construct with biobricks for alkane degradation as described by the project description.
The highest calculated theoretical maximal yield for biomass in Cmol per Cmol is achieved with an odd alkane with 9 carbon atoms. Here the yield is 0.853 Cmol of biomass per Cmol of substrate. For even alkanes the theoretical maximal biomass yield is constant at 0.788 Cmol biomass per Cmol substrate. For glucose this value is 0.775 Cmol biomass per Cmol substrate. With the optimazation all the carbon atoms go to biomass and CO2, so the CO2 production is the highest for glucose and the lowest for odd alkanes.
The optimal alkane degradation does however consume more than three times as much oxygen than growth on glucose. Oxygen is often a limiting factor for growth and especially in oily environments the oxygen transfers less easily into the water phase. This additional oxygen consumption originates from the first step in the alkane degradation, which consumes oxygen and the large amount of cofactors that need to be regenerated, which can be seen from the high ATP surplus in the bottom right graph of figure 1. This large surplus of energy is just wasted because of the optimazation, but in reality this energy may be used for something useful.
NO3 as electron acceptor
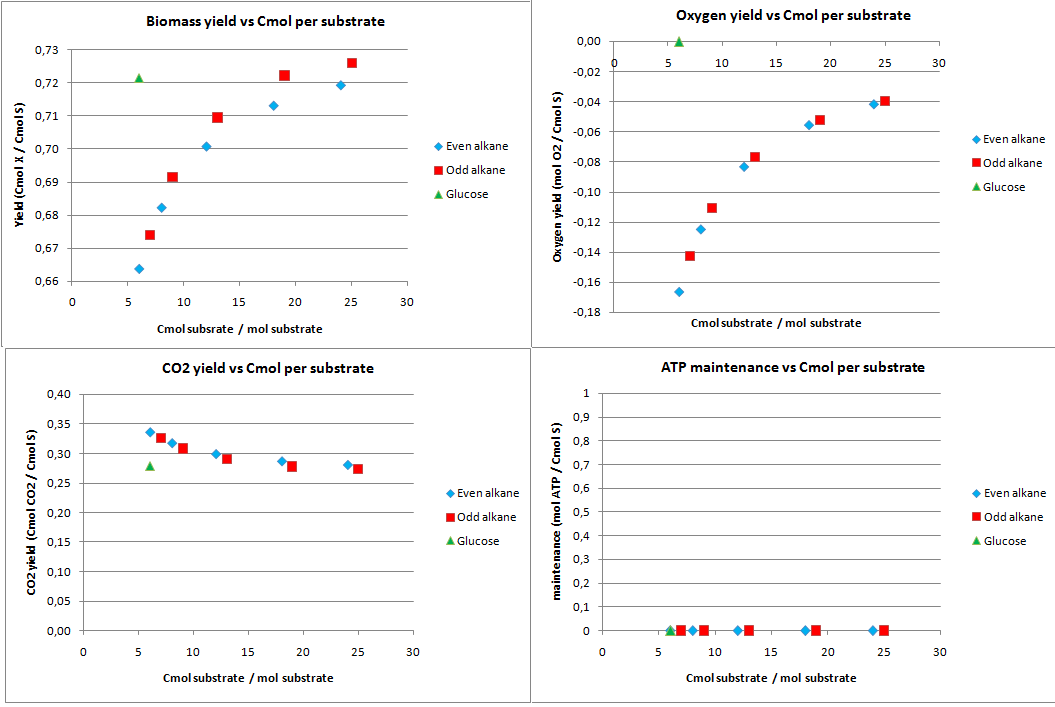
This construct was introduced to see how much the oxygen consumption could theoretically be eliminated with growth on alkanes. In oily environments oxygen transfers poorly into the water phase. The yields for the optimazation to growth with NO3 as electron acceptor are displayed in figure 2.
The highest theoretical biomass yield is achieved with an odd alkane with 25 carbon atoms. The yield for biomass on glucose is only slighlty lower. Growth on short-chained alkanes has clearly a lower yield, dropping by 10% for the shortest alkanes.
The oxygen consumption for growth on glucose is completely eliminated and for the alkanes the oxygen consumption drops as the chain length increases. In this scenario the cell has no energy left over and the maintenance is zero.
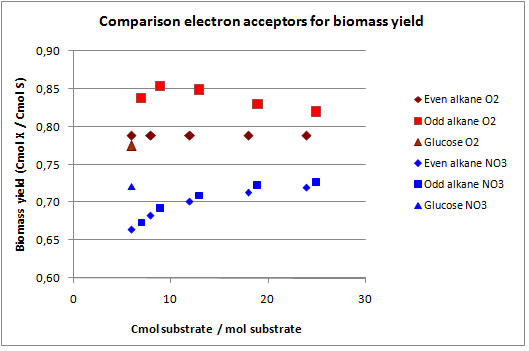
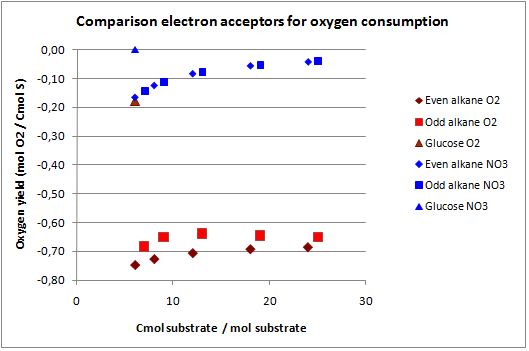
In figures 3 and 4 the previous scenario, the standard scenario with oxygen as electron acceptor, and the current scenario are compared in terms of biomass yield and oxygen requirement.
With NO3 as electron acceptor less ATP is produced per NADH and FADH2. This translates into a lower biomass yield as observed. The biomass yield drops 9% to 19% for the alkanes and 7% for glucose. The oxygen requirement however drops 77% to 94% for the alkanes and is completely eliminated for glucose. Overexpressing this system may be very benificial in oily environments.
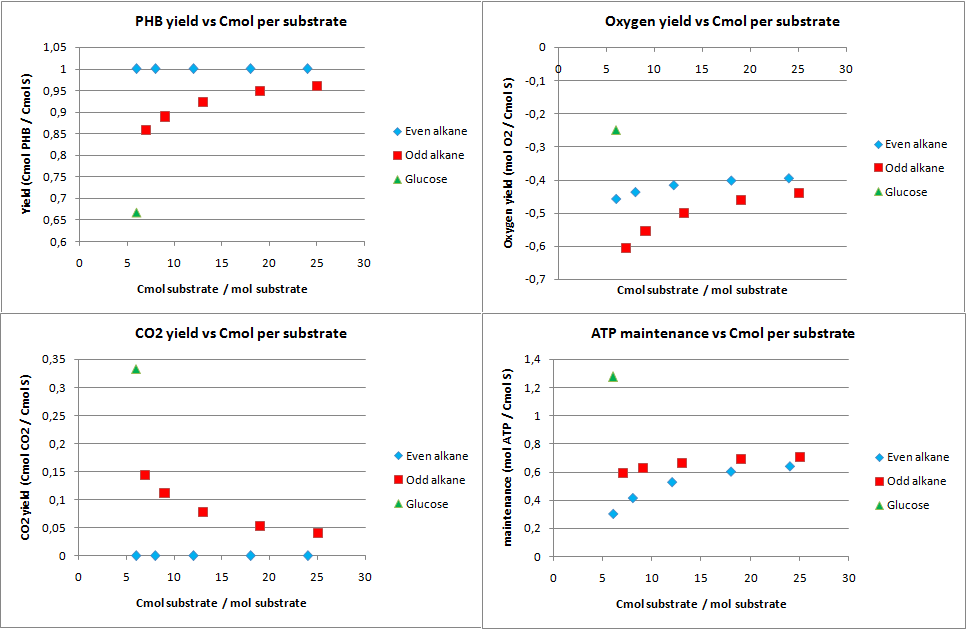
PHB production
This scenario was proposed to see how well the alkane degradation could be used to produce a useful substance instead of biomass and CO2. The yields from the analysis for this scenario are displayed in figure 5.
For any even alkane the yield is 1 Cmol of PHB per Cmol of substrate. This is the highest achievable yield without any other carbon source. The CO2 for even alkanes is therefore also zero, as all carbon atoms go into the product. Odd numbered alkanes also have very high yields of 0.85 to 0.95 Cmol PHB per Cmol substrate. Glucose has a yield of 0.667 Cmol PHB per Cmol of glucose. This lower yield can be explained by the fact that the the PHB production pathway starts at a C2 molecule. Glucose splits into two C3 moleucules which are then converted into C2 molecules by producing CO2. This generation of CO2 explaines both the lower yields for glucose and odd numbered alkanes. Odd numbered alkanes produce propionyl-CoA, which is also a C3 molecule.
The system generates a lot of energy, the ATP left over is very high. This allows for growth and other options for the cell if the yield for PHB is less then theoretically maximal.
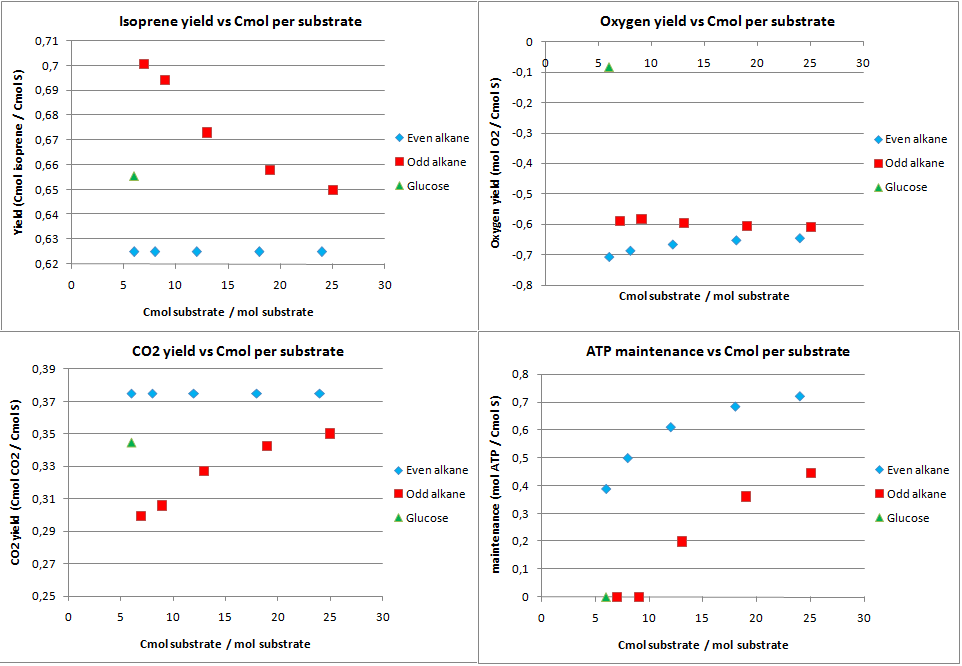
isoprene production
Isoprene production was chosen as a hypothetical pathway. Although it is an unlikely product for E. coli, in theory it holds much promise as it is very reduced. The calculated yields from the metabolic flux analysis are displayed in figure 6.
The product yields for isoprene are lower than for PHB in the previous case. The upper limit is only 0.833 Cmol isoprene per Cmol substrate, as in the first step of the isoprene pathway 2 C3 molecules are used to form a C5 molecule and CO2. The yields for isoprene on alkanes are further lowered because the acetyl-CoA (C2) needs to be converted to C3 molecules. This leads to CO2 production through the anapleorotic routes and therefore in a drop of the yield. This also why short-chained odd numbered alkanes have a higher yield as the ration propionyl-CoA/acetyl-CoA is higher for shorter odd numbered alkanes. The yield for glucose is lower than the absolute maximum because substrate needs to be consumed for production of cofactors that are required for the isoprene pathway. This is also relfected by the fact that no ATP is going to maintenance with glucose as substrate.
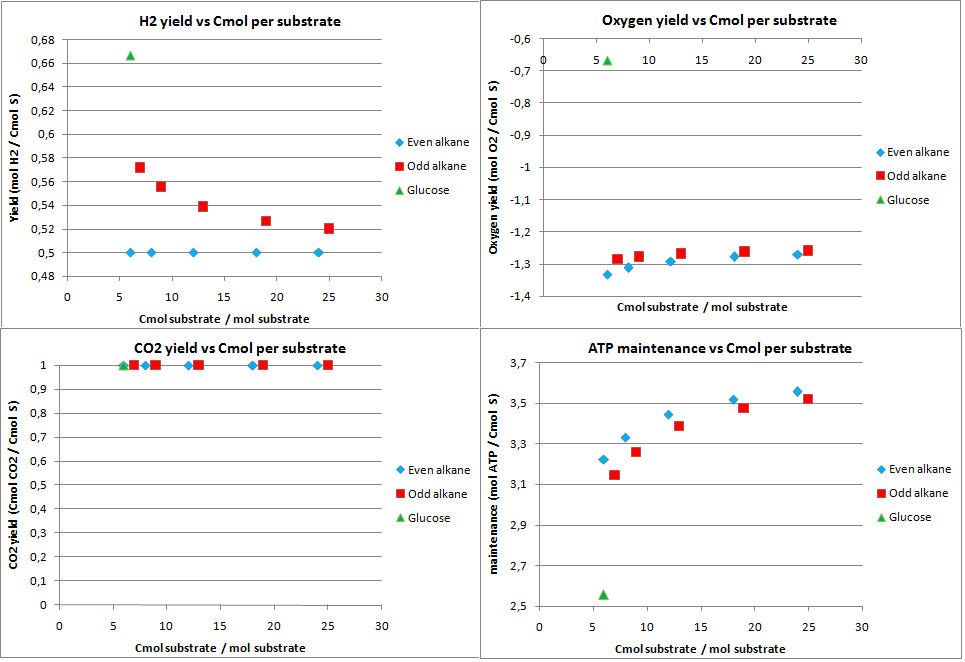
hydrogen production
 "
"