Team:TU Delft/21 July 2010 content
From 2010.igem.org
(Difference between revisions)
(→Ordered DNA) |
|||
| Line 17: | Line 17: | ||
|'''Primer''' | |'''Primer''' | ||
|'''Status''' | |'''Status''' | ||
| - | |||
|- | |- | ||
|M1 | |M1 | ||
| Line 24: | Line 23: | ||
|n/a | |n/a | ||
|n/a | |n/a | ||
| - | |||
|- | |- | ||
|1 | |1 | ||
| Line 31: | Line 29: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=limegreen>✓</font> | |<font color=limegreen>✓</font> | ||
| - | |||
|- | |- | ||
|2 | |2 | ||
| Line 38: | Line 35: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|- | |- | ||
|3 | |3 | ||
| Line 45: | Line 41: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|- | |- | ||
|4 | |4 | ||
| Line 52: | Line 47: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|- | |- | ||
|5 | |5 | ||
| Line 59: | Line 53: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|- | |- | ||
|6 | |6 | ||
| Line 66: | Line 59: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|- | |- | ||
|7 | |7 | ||
| Line 73: | Line 65: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|- | |- | ||
|8 | |8 | ||
| Line 80: | Line 71: | ||
|G00100 + G00101 | |G00100 + G00101 | ||
|<font color=red>✗</font> | |<font color=red>✗</font> | ||
| - | |||
|} | |} | ||
Unfortunately all other lanes were empty or the same as the negative control. So from this gel we conclude that the ligation has failed. | Unfortunately all other lanes were empty or the same as the negative control. So from this gel we conclude that the ligation has failed. | ||
Revision as of 20:26, 5 August 2010
Contents |
Lab work
Ordered DNA
The transformations of 19 July containing the different ligations gave colonies (~5 per plate). We picked 5 colonies per plate and performed a colony PCR.
Alkane degradation
Unfortunately there were no transformants on yesterday's plates. This is most likely due to the fact that the ligation didn't work with the small pieces of DNA of the RBSs. Next plan is to cut open the RBS plasmid without removing the RBS (with SpeI and PstI) and insert the gene in this plasmid. We will try this tomorrow.
Emulsifier
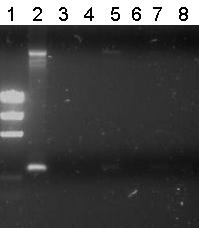
There were small colonies on the plates. Pieter picked seven and performed colony PCR on them.
Lane Description
| # | Description | Expected lenght (bp) | Primer | Status |
| M1 | EZ Ladder | n/a | n/a | n/a |
| 1 | B0032 (control) | 220 | G00100 + G00101 | ✓ |
| 2 | Transformant #1 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 3 | Transformant #2 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 4 | Transformant #3 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 5 | Transformant #4 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 6 | Transformant #5 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 7 | Transformant #6 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 8 | Transformant #7 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
Unfortunately all other lanes were empty or the same as the negative control. So from this gel we conclude that the ligation has failed.
 "
"