Team:TU Delft/15 July 2010 content
From 2010.igem.org
| Line 2: | Line 2: | ||
<h4>Ordered DNA + Solvent Tolerance and Hydrocarbon Sensing</h4> | <h4>Ordered DNA + Solvent Tolerance and Hydrocarbon Sensing</h4> | ||
| - | Some of transformations of [https://2010.igem.org/Team:TU_Delft#/blog?blog=14_July_2010 yesterday] containing the different ligations gave colonies (1 a 2 per plate). We picked as many colonies as possible of every plate and performed a [[Team:TU_Delft/protocols/colony PCR|colony PCR]] to check which colonies contained the right insert. At the same time we grow them overnight in 5 mL LB medium containing the appropriate antibiotic. | + | Some of transformations of [https://2010.igem.org/Team:TU_Delft#/blog?blog=14_July_2010 yesterday] containing the different ligations gave colonies (1 a 2 per plate). We picked as many colonies as possible of every plate and performed a [[Team:TU_Delft/protocols/colony PCR|colony PCR]] overnight to check which colonies contained the right insert. At the same time we grow them overnight in 5 mL LB medium containing the appropriate antibiotic. |
===Emulsifier=== | ===Emulsifier=== | ||
| - | The results from the Colony PCR were not conclusive [https://2010.igem.org/Team:TU_Delft#/blog?blog=14_July_2010 yesterday]. That is why we decided to | + | The results from the Colony PCR were not conclusive [https://2010.igem.org/Team:TU_Delft#/blog?blog=14_July_2010 yesterday]. That is why we decided to isolate the plasmid using [[Team:TU_Delft/protocols/birnboim_plasmid_isolation|Birnboim protocol]]. |
| - | + | ||
| - | The | + | The following plasmid concentrations were obtained: |
| + | {| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| + | |'''BioBrick''' | ||
| + | |'''Composed of''' | ||
| + | |'''Concentration (ng/μL)''' | ||
| + | |- | ||
| + | |[http://partsregistry.org/wiki/index.php?title=Part:BBa_K398203 K398203] | ||
| + | | OprG-B0015 (transformant 6) | ||
| + | | | ||
| + | |- | ||
| + | |[http://partsregistry.org/wiki/index.php?title=Part:BBa_K398203 K398203] | ||
| + | | OprG-B0015 (transformant 7) | ||
| + | | | ||
| + | |- | ||
| + | |[http://partsregistry.org/wiki/index.php?title=Part:BBa_K398204 K398204] | ||
| + | | AlnA-B0015 (transformant 11) | ||
| + | | | ||
| + | |} | ||
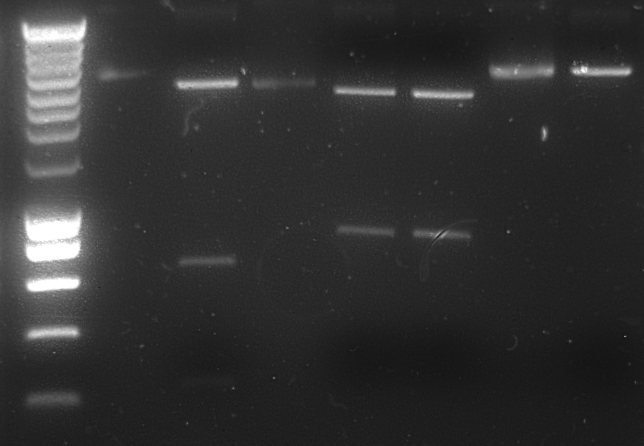
| - | [[ | + | The plasmids were subsequently [[Team:TU_Delft/protocols/restriction_enzyme_digestion|digested]] with an enzyme that characteristic digest for all different plasmids. The plasmids were digested with HindIII. This should yield 3 bands in the lane with the plasmid pSB1T3 containing J04450 (neg control), 1 band in the lane with AlnA-B0015 and 2 bands in the lane with OprG-B0015 on gel. |
{| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | {| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| Line 32: | Line 49: | ||
|Buffer 2 + BSA (BioLabs) | |Buffer 2 + BSA (BioLabs) | ||
|} | |} | ||
| + | |||
| + | [[Team:TU_Delft/protocols/agarose_gel |1% Agarose gel]] of digestion products: | ||
| + | [[Image:TU_Delft_2010-07-15_Digestion.png|300px|thumb|left|1% Agarose gel of digestion products]] | ||
Lane description: | Lane description: | ||
Revision as of 19:47, 19 July 2010
Lab work
Ordered DNA + Solvent Tolerance and Hydrocarbon Sensing
Some of transformations of yesterday containing the different ligations gave colonies (1 a 2 per plate). We picked as many colonies as possible of every plate and performed a colony PCR overnight to check which colonies contained the right insert. At the same time we grow them overnight in 5 mL LB medium containing the appropriate antibiotic.
Emulsifier
The results from the Colony PCR were not conclusive yesterday. That is why we decided to isolate the plasmid using Birnboim protocol.
The following plasmid concentrations were obtained:
| BioBrick | Composed of | Concentration (ng/μL) |
| K398203 | OprG-B0015 (transformant 6) | |
| K398203 | OprG-B0015 (transformant 7) | |
| K398204 | AlnA-B0015 (transformant 11) |
The plasmids were subsequently digested with an enzyme that characteristic digest for all different plasmids. The plasmids were digested with HindIII. This should yield 3 bands in the lane with the plasmid pSB1T3 containing J04450 (neg control), 1 band in the lane with AlnA-B0015 and 2 bands in the lane with OprG-B0015 on gel.
| # | Digestion reaction | Used Buffer |
| 1 | 1.5 μg isolated control plasmid pSB1T3 | Buffer 2 + BSA (BioLabs) |
| 2 | 1 μg isolated plasmid with OprG (culture 6) | Buffer 2 + BSA (BioLabs) |
| 3 | 1 μg isolated plasmid with OprG (culture 7) | Buffer 2 + BSA (BioLabs) |
| 4 | 1 μg isolated plasmid with AlnA | Buffer 2 + BSA (BioLabs) |
1% Agarose gel of digestion products:
Lane description:
| # | Description | Expected Length (bp) |
| 1 | SmarLadder marker (5 μL) | |
| 2 | Undigested control plasmid (5 μL + 1 μL loadingbuffer) | |
| 3 | Digested control plasmid (10 μL + 1 μL loadingbuffer) | 1972, 1176 |
| 4 | Undigested culture 6 OprG (5 μL + 1 μL loadingbuffer) | |
| 5 | Digested culture 6 OprG (10 μL + 2 μL loadingbuffer) | 1828, 1475, 222 |
| 6 | Digested culture 7 OprG (10 μL + 2 μL loadingbuffer) | 1828, 1475, 222 |
| 7 | Undigested culture 11 AlnA (5 μL + 1 μL loadingbuffer) | |
| 8 | Digested culture 11 AlnA (10 μL + 2 μL loadingbuffer) | 3511 |
 "
"