Team:Stockholm/Project Idea/Proteins
From 2010.igem.org
(→Proteins) |
|||
| Line 6: | Line 6: | ||
== Proteins == | == Proteins == | ||
| - | + | === Superoxide dismutase 1 protein, SOD === | |
| + | [http://partsregistry.org/Part:BBa_K380007 Part:BBa_K380007] | ||
| + | |||
| + | |||
| + | Human soluble superoxide dismutase 1 (SOD1) is a soluble cytoplasmic protein functional as a homodimer that binds copper and zink ions. SOD1 catalyzes the reaction O<sup>-</sup><sub>2</sub> + O<sup>-</sup><sub>2</sub> + 2H<sup>+</sup> → H<sub>2</sub>O<sub>2</sub> + O<sub>2</sub>, protecting the cell from oxidative damage. SOD1 was first cloned and expressed in E. coli by [http://www.ncbi.nlm.nih.gov/pubmed/3889846 Hallewell ''et al''., (1985)]. | ||
| + | |||
| + | [[Image:SOD1_dimeric.png|400px|thumb|right|3D structure of human SOD1 in it's dimeric form. Primary citation [http://www.ncbi.nlm.nih.gov/pubmed/20822138 Leinartaite ''et al''. (2010)] ]] | ||
| + | |||
| + | {| | ||
| + | | '''Gene''' (cDNA) | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 465 bp | ||
| + | |- | ||
| + | | removed restriction site | ||
| + | | PfeI | ||
| + | |- | ||
| + | | exchanged nt | ||
| + | | nt331 A → G | ||
| + | |- | ||
| + | | '''Protein''' | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 154 aa | ||
| + | |- | ||
| + | | size | ||
| + | | 15,936 Da | ||
| + | |- | ||
| + | | Fasta | ||
| + | | [http://www.ncbi.nlm.nih.gov/protein/49456443?report=fasta SOD1] | ||
| + | |} | ||
| + | |||
| + | |||
| + | GenBank:[http://www.ncbi.nlm.nih.gov/nucleotide/38489879?report=genbank&log$=nucltop&blast_rank=22&RID=CAM83NYN01S AY450286.1] | ||
| + | |||
| + | First reported by:[http://www.ncbi.nlm.nih.gov/pubmed/3889846 Hallewell ''et al''., (1985)]. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | === copper chaperon protein for SOD, yCCS === | ||
| + | [http://partsregistry.org/Part:BBa_K380008 Part:BBa_K380008] | ||
| + | |||
| + | |||
| + | Yeast copper chaperon protein (yCCS) is a help chaperon specific for copper/zinc superoxide dismutase located to the cytoplasm. yCCS generates fully metallized, active SOD1 proteins that in turn protects the cell from oxidative damage. | ||
| + | |||
| + | yCCS has been shown to successfully mediate the delivery of copper ions to human SOD1 ([http://www.ncbi.nlm.nih.gov/pubmed/15358352 Ahl ''et al''. 2003]). Co-expression of SOD1 and yCCS yields proteins with higher copper contents, leading to increased activity and more stable proteins. | ||
| + | |||
| + | [[Image:YSOD+yCCS_interaction.jpg|400px|thumb|right|3D structure of yCCS interacting with yeast superoxide dismutase (ySOD) in it's monomeric form. Ions indicated as gray orbs. Primary citation [[http://www.ncbi.nlm.nih.gov/pubmed/11524675 Lamb ''et al''. 2001]] ]] | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | | '''Gene''' (cDNA) | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 750bp | ||
| + | |- | ||
| + | | removed restriction site | ||
| + | | EcoRI | ||
| + | |- | ||
| + | | exchanged nt | ||
| + | | nt257 T → C | ||
| + | |- | ||
| + | | '''Protein''' | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 249 aa | ||
| + | |- | ||
| + | | size | ||
| + | | 27,330 Da | ||
| + | |- | ||
| + | | Fasta | ||
| + | | [http://www.ncbi.nlm.nih.gov/protein/596088?report=fasta yCCS] | ||
| + | |} | ||
| + | |||
| + | |||
| + | GenBank:[http://www.ncbi.nlm.nih.gov/nuccore/NM_001182535.1?report=genbank&log$=seqview NM_001182535.1] | ||
| + | |||
| + | First reported by:[http://www.ncbi.nlm.nih.gov/pubmed/9295278 Culotta ''et al''. (1997)]. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | |||
| + | |||
| + | === basic fibroblast growth factor, bFGF === | ||
| + | [http://partsregistry.org/Part:BBa_K380006 Part:BBa_K380006] | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:BFGF.jpg|200px|thumb|right|3D structure of bFGF. Primary citation [http://www.ncbi.nlm.nih.gov/pubmed/20133753 Bae ''et al''. 2010] ]] | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | | '''Gene''' (cDNA) | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 468 bp | ||
| + | |- | ||
| + | | removed restriction sites | ||
| + | | AgeI | ||
| + | |- | ||
| + | | exchanged nt | ||
| + | | | ||
| + | |- | ||
| + | | '''Protein''' | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 288 aa | ||
| + | |- | ||
| + | | size | ||
| + | | 30,770 Da | ||
| + | |- | ||
| + | | Fasta | ||
| + | | [http://www.ncbi.nlm.nih.gov/protein/153285461?report=fasta bFGF] | ||
| + | |} | ||
| + | |||
| + | |||
| + | GenBank: | ||
| + | |||
| + | First reported by: | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | |||
| + | |||
| + | === Protein A, z domain === | ||
| + | [http://partsregistry.org/Part:BBa_K380009 Part:BBa_K380009] | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:|300px|thumb|right|]] | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | | '''Genepart''' | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 174 bp | ||
| + | |- | ||
| + | | removed restriction sites | ||
| + | | - | ||
| + | |- | ||
| + | | exchanged nt | ||
| + | | - | ||
| + | |- | ||
| + | | '''Protein''' | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 58 aa | ||
| + | |- | ||
| + | | size | ||
| + | | | ||
| + | |- | ||
| + | | Fasta | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | |||
| + | GenBank: | ||
| + | |||
| + | First reported by: | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | |||
| + | |||
| + | === IgG protease, IdeS === | ||
| + | [http://partsregistry.org/Part:BBa_K380010 Part:BBa_K380010] | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:IdeS.jpg|200px|thumb|right|3D structure of IdeS. Primary citation [http://www.ncbi.nlm.nih.gov/pubmed/15574492 Wenig ''et al''. 2004] ]] | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | | '''Gene''' (cDNA) | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 930 bp | ||
| + | |- | ||
| + | | removed restriction sites | ||
| + | | - | ||
| + | |- | ||
| + | | exchanged nt | ||
| + | | - | ||
| + | |- | ||
| + | | '''Protein''' | ||
| + | | | ||
| + | |- | ||
| + | | length | ||
| + | | 339 aa | ||
| + | |- | ||
| + | | size | ||
| + | | 37,977 Da | ||
| + | |- | ||
| + | | Fasta | ||
| + | | [http://www.ncbi.nlm.nih.gov/protein/209559219?report=fasta IdeS] | ||
| + | |} | ||
| + | |||
| + | |||
| + | GenBank: | ||
| + | |||
| + | First reported by: | ||
| + | |||
| + | |||
| + | |||
| + | |||
|} | |} | ||
{{Stockholm/Footer}} | {{Stockholm/Footer}} | ||
Revision as of 23:59, 26 October 2010
ProteinsSuperoxide dismutase 1 protein, SOD
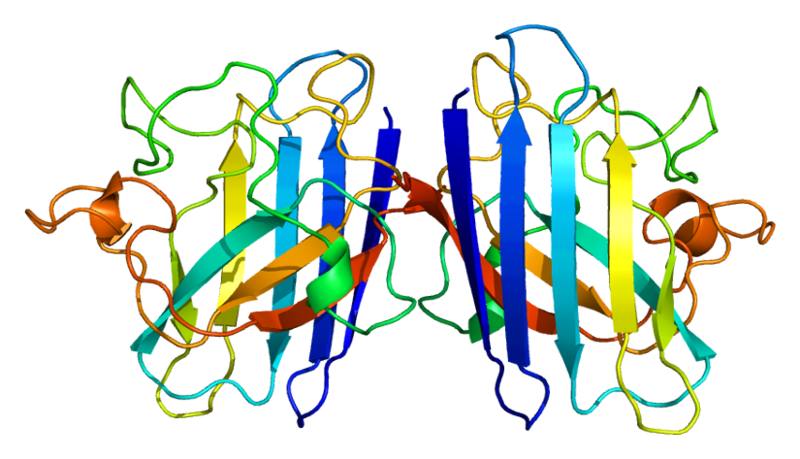 3D structure of human SOD1 in it's dimeric form. Primary citation Leinartaite et al. (2010)
First reported by:Hallewell et al., (1985).
copper chaperon protein for SOD, yCCS
yCCS has been shown to successfully mediate the delivery of copper ions to human SOD1 (Ahl et al. 2003). Co-expression of SOD1 and yCCS yields proteins with higher copper contents, leading to increased activity and more stable proteins.  3D structure of yCCS interacting with yeast superoxide dismutase (ySOD) in it's monomeric form. Ions indicated as gray orbs. Primary citation [Lamb et al. 2001]
First reported by:Culotta et al. (1997).
basic fibroblast growth factor, bFGF
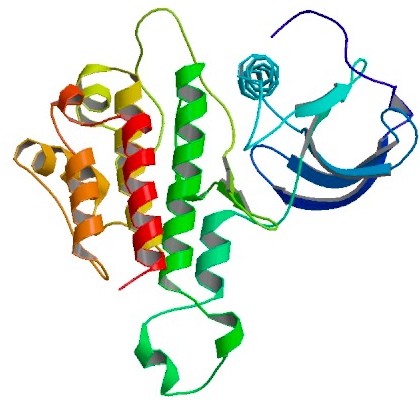 3D structure of bFGF. Primary citation Bae et al. 2010
First reported by:
Protein A, z domain
[[Image:|300px|thumb|right|]]
First reported by:
IgG protease, IdeS
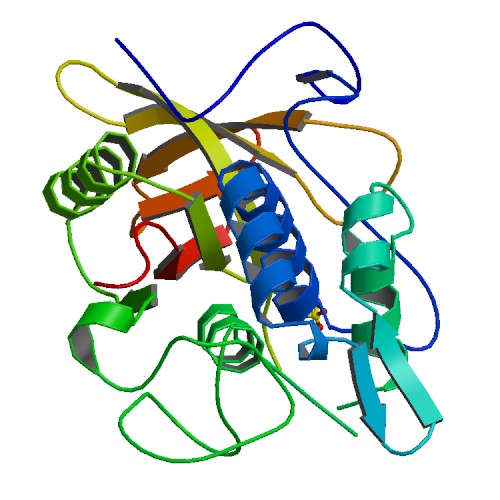 3D structure of IdeS. Primary citation Wenig et al. 2004
First reported by:
|
 |

|
 |

|
 |

|

|

|
 "
"


