Team:Stockholm/27 July 2010
From 2010.igem.org
m |
|||
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Stockholm/Top2}} | {{Stockholm/Top2}} | ||
| + | |||
== Mimmi == | == Mimmi == | ||
| Line 9: | Line 10: | ||
| - | {| border="1" cellspacing="0 | + | {| border="1" cellspacing="0" |
|- | |- | ||
| - | ! | + | ! Mix |
| - | + | | v | |
|- | |- | ||
| - | + | | LB | |
| 4 ml | | 4 ml | ||
|- | |- | ||
| - | + | | glycose | |
| 40 µl (1%) | | 40 µl (1%) | ||
|- | |- | ||
| - | + | | Amp | |
| 8 µl (0.5%) | | 8 µl (0.5%) | ||
|- | |- | ||
| - | + | | old culture | |
| 40 µl | | 40 µl | ||
|} | |} | ||
| Line 48: | Line 49: | ||
::::(MW/10)/125 = df to get 125 ng/µl | ::::(MW/10)/125 = df to get 125 ng/µl | ||
| - | ::{| border="1" cellspacing="0 | + | ::{| border="1" cellspacing="0" |
|- | |- | ||
| - | ! | + | ! primer |
| - | ! | + | ! V H2O (µl) |
| - | ! | + | ! V H2O to get 125 ng/µl (µl) |
|- | |- | ||
| - | + | | MITF_site1_F | |
| 463.88 | | 463.88 | ||
| 3µl + 15.4µl | | 3µl + 15.4µl | ||
|- | |- | ||
| - | + | | MITF_site1_R | |
| 64.77 | | 64.77 | ||
| 3µl + 15.3µl | | 3µl + 15.3µl | ||
|- | |- | ||
| - | + | | MITF_site2_F | |
| 144.85 | | 144.85 | ||
| 3µl + 21µl | | 3µl + 21µl | ||
|- | |- | ||
| - | + | | MITF_site2_R | |
| 141.77 | | 141.77 | ||
| 3µl + 21.6µl | | 3µl + 21.6µl | ||
|- | |- | ||
| - | + | | | |
| - | ! | + | ! concentration |
| - | + | | | |
|- | |- | ||
| - | + | | yCCS_F | |
| 1085.7 ng/µl | | 1085.7 ng/µl | ||
| 3µl + 23µl | | 3µl + 23µl | ||
|- | |- | ||
| - | + | | yCCS_R | |
| 996.5 ng/µl | | 996.5 ng/µl | ||
| 3µl + 20.9µl | | 3µl + 20.9µl | ||
| Line 85: | Line 86: | ||
==== reaction ==== | ==== reaction ==== | ||
| - | {| border="1" cellspacing="0 | + | {| border="1" cellspacing="0" |
|- | |- | ||
| - | ! | + | ! Mix |
| - | + | | width="50" | (µl) | |
| - | + | | width="50" |X2 | |
|- | |- | ||
| - | + | | H2O | |
| 40 | | 40 | ||
| 80 | | 80 | ||
|- | |- | ||
| - | + | | dNTPs | |
| 1 | | 1 | ||
| 2 | | 2 | ||
|- | |- | ||
| - | + | | F primer | |
| 1 | | 1 | ||
| 2 | | 2 | ||
|- | |- | ||
| - | + | | R primer | |
| 1 | | 1 | ||
| 2 | | 2 | ||
|- | |- | ||
| - | + | | DNA | |
| 1 | | 1 | ||
| 2*1 | | 2*1 | ||
|- | |- | ||
| - | + | | Pfu X10 buffer | |
| 5 | | 5 | ||
| 10 | | 10 | ||
|- | |- | ||
| - | + | | Pfu turbo pol | |
| 1 | | 1 | ||
| 2 | | 2 | ||
| Line 177: | Line 178: | ||
*Plate the 100µl on a Amp-plate | *Plate the 100µl on a Amp-plate | ||
*Grow ON at 37°C | *Grow ON at 37°C | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==Hassan== | ==Hassan== | ||
| Line 191: | Line 187: | ||
---- | ---- | ||
| - | [ | + | [http://string.embl.de/version_8_3/newstring_cgi/show_network_section.pl?identifiers=9606.ENSP00000331746%250D9606.ENSP00000295600%250D9606.ENSP00000364016%250D9606.ENSP00000371175%250D9606.ENSP00000269280%250D9606.ENSP00000363700%250D9606.ENSP00000360157%250D9606.ENSP00000373571%250D9606.ENSP00000291700&channel1=off&channel2=off&channel3=off&channel4=on&channel5=on&channel6=on&channel7=on&interactive=yes&network_flavor=actions&targetmode=proteins] |
| - | [ | + | [http://string.embl.de/version_8_3/newstring_cgi/show_network_section.pl?identifiers=9606.ENSP00000331746%250D9606.ENSP00000295600%250D9606.ENSP00000226877%250D9606.ENSP00000371175%250D9606.ENSP00000269280%250D9606.ENSP00000363700%250D9606.ENSP00000360157%250D9606.ENSP00000373571%250D9606.ENSP00000291700&channel1=off&channel2=off&channel3=off&channel4=on&channel5=on&channel6=on&channel7=on&additional_network_nodes=5&interactive=yes&network_flavor=actions&targetmode=proteins] |
| Line 289: | Line 285: | ||
---- | ---- | ||
| - | |||
==Nina== | ==Nina== | ||
| Line 364: | Line 359: | ||
33: ASB0045 103 | 33: ASB0045 103 | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Latest revision as of 10:56, 26 October 2010
Contents |
Mimmi
over-expression in pEX
- - remaking the comassie-gel
| Mix | v |
|---|---|
| LB | 4 ml |
| glycose | 40 µl (1%) |
| Amp | 8 µl (0.5%) |
| old culture | 40 µl |
- Grow in 37C, ~200 rpm ~2h until OD = 0.6
- Add IPTG (1 µl/ml,1M)in one of the two cultures, the other one is used as a control
- Take sample at 0h, 1h, 2h, 3h
- pipette 500µl into a 1.5ml tube
- spinn down the sells and remove LB
- resuspend in 50µl loading dye
- freeze
Site-Directed Mutagenesis
Primers
- (MW/10)/(OD*33) = dilution factor
- 1000/df = volyme to get 100µM primer
- 100µM = (MW/10) ng/µl
- (MW/10)/125 = df to get 125 ng/µl
- 100µM = (MW/10) ng/µl
- 1000/df = volyme to get 100µM primer
primer V H2O (µl) V H2O to get 125 ng/µl (µl) MITF_site1_F 463.88 3µl + 15.4µl MITF_site1_R 64.77 3µl + 15.3µl MITF_site2_F 144.85 3µl + 21µl MITF_site2_R 141.77 3µl + 21.6µl concentration yCCS_F 1085.7 ng/µl 3µl + 23µl yCCS_R 996.5 ng/µl 3µl + 20.9µl
reaction
| Mix | (µl) | X2 |
|---|---|---|
| H2O | 40 | 80 |
| dNTPs | 1 | 2 |
| F primer | 1 | 2 |
| R primer | 1 | 2 |
| DNA | 1 | 2*1 |
| Pfu X10 buffer | 5 | 10 |
| Pfu turbo pol | 1 | 2 |
MITF yCCS conditions MITF_Site1_F yCCS_F time °C MITF_Site2_R yCCS_R 30s 95 pRc/CMV pSB1C3 30s 95 ~6.7kb ~3,5kb 30s 55 7m 68 oo 4
- Make sure sample is ≤ 37°C
- Add 1µl Dpn1 (to 50µl product) and incubate in 37°C ON
Transformation
- mix
- Top 10 competent cells 100µl
- pRc/CMV.MITF_M 1µl
- Hold on ice 30min
- Heat shock 42°C, 55sec
- Cool down 1min (on ice)
- Add 900µl LB
- Incubate 37°C, 250rpm, 1h (forgot rpm)
- Spinn down cells, 13000rpm, 15sec
- Remove 900µl LB
- Plate the 100µl on a Amp-plate
- Grow ON at 37°C
Hassan
Nina
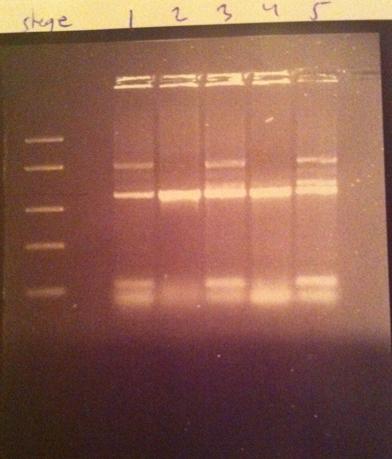
Colony PCR on IgG protease in shipping vector
I made a colony PCR on the IgG protease that I have inserted into the iGEM shipping vector to verify that the gene is inserted in a correct position. Therefore I used one of the gene's primers and one of the vector's verification primers. The colony numbers are: 1, 2, 3, 4 and 5.
PCR reaction mix:
- 1 µl Morten's polymerase PjuX7
- 1 µl 10 mM dNTPs
- 3 µl 5 µM forward primer (VF2)
- 3 µl 5 µM revers primer (gene's primer)
- 10 µl buffer 5X
- 1 µl MgCl2 50mM
- 30 µl H2O
- DNA template was one colony
PCR program:
98°C - 2 min
31 cycles of:
- 98°C - 10 sec
- 55°C - 15 sec
- 72°C - 1.5 min
72°C - 5 min
4°C - ∞
DNA Ladder: FastRuler™ Middle Range, ready-to-use, 100-5000 bp Fermentas
Colony number 2 looks good on the gel. This one will be inoculated in LB in order to become minipreped to be shipped to iGEM hq.
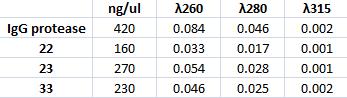
Mini prep on IgG protease and CPP
I prepare a miniprep on the inoculated IgG protease with colony number 2. In addition I miniprep three inoculated samples with vector carrying CPP from colony number 22, 23 and 33.
The method is carried out according to the procedure in protocols.
Measuring concentration with spectrophotometer:
Sequencing CPP TAT N version
I send three samples of CPP TAT N version for sequencing. Colony numbers are: 22, 23 and 33.
- 15 ul vector
- 1.5 ul 10uM VR2 primer
22: ASB0045 105
23: ASB0045 104
33: ASB0045 103
 |

|
 |

|
 |

|

|

|
 "
"