Team:Slovenia/PROJECT/proof/studies/emsa
From 2010.igem.org
m |
|||
| Line 77: | Line 77: | ||
<h2>Introduction</h2> | <h2>Introduction</h2> | ||
| - | Electrophoretic mobility shift assay (EMSA) is a technique for studying protein-DNA interactions <em>in vitro</em>. The underlying principle lies in sequence specific binding of particular proteins to a defined double straned DNA target. The complex between DNA and protein has different mobility than DNA or | + | Electrophoretic mobility shift assay (EMSA) is a technique for studying protein-DNA interactions <em>in vitro</em>. The underlying principle lies in sequence specific binding of particular proteins to a defined double straned DNA target. The complex between DNA and protein has different mobility than DNA or protein alone and ise detected on the electrophoretic gel. The principle is as follows: purified proteins are incubated with DNA target sequence and samples are then loaded on an agarose gel prestained with ethidium bromide which is able to intercalate/bind to DNA. Ethidium bromide can be visualized under UV light, allowing to observe migration of DNA with time. If a protein of interest binds to DNA, this protein-DNA complex, being much larger than DNA itself, is shifted as a consequence of different charge/hydrodynamic radius ratio. We can observe either DNA or protein of the complex. Net charge of a protein is also important. Since DNA is negatively charged, protein usually needs to be basic at neutral pH in order to bind DNA. We used this assay as an additionally prove of binding of zinc fingers to DNA targets. |
<h2>Results</h2> | <h2>Results</h2> | ||
Latest revision as of 01:53, 28 October 2010
Contents |
Introduction
Electrophoretic mobility shift assay (EMSA) is a technique for studying protein-DNA interactions in vitro. The underlying principle lies in sequence specific binding of particular proteins to a defined double straned DNA target. The complex between DNA and protein has different mobility than DNA or protein alone and ise detected on the electrophoretic gel. The principle is as follows: purified proteins are incubated with DNA target sequence and samples are then loaded on an agarose gel prestained with ethidium bromide which is able to intercalate/bind to DNA. Ethidium bromide can be visualized under UV light, allowing to observe migration of DNA with time. If a protein of interest binds to DNA, this protein-DNA complex, being much larger than DNA itself, is shifted as a consequence of different charge/hydrodynamic radius ratio. We can observe either DNA or protein of the complex. Net charge of a protein is also important. Since DNA is negatively charged, protein usually needs to be basic at neutral pH in order to bind DNA. We used this assay as an additionally prove of binding of zinc fingers to DNA targets.
Results
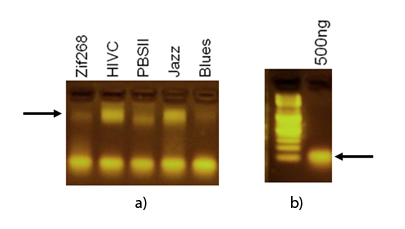
Specific DNA binding of synthetic zinc finger domains to the program nucleic acid was tested by EMSA. 1 µg of purified proteins were incubated with 500 ng of program nucleic acid. Samples diluted with high grade laboratory water to 20µl were loaded on a 2.0% agarose gel prestained with ethidium bromide and run at 70 V for 40 minutes. Nucleic acid – protein – complexes were detected under UV light. Results clearly demonstrate the difference in mobility between protein-DNA complexes and unbound/free DNA target.
 "
"