Team:Slovenia/PROJECT/proof/studies/betagal
From 2010.igem.org
Contents |
Introduction
Before we started working on our main project (biosynthesis), we had to make sure the zinc finger proteins actually bind to their predicted binding sites. To evaluate binding of zinc finger-enzyme fusion proteins in vitro, we used different methods and techniques, e.g surface plasmon resonance (SPR) and electrophoretic mobility shift assay (EMSA). In addition, we designed a universal device to measure the binding of DNA binding proteins to the specific DNA sequences in vivo (scheme below).
The universal device for testing in vivo binding of DNA binding proteins is composed of several parts (see the scheme): 1) a synthetic promoter, pSYN, into which a DNA binding sequence, specific for each tested DNA binding protein was inserted (between -35 and -10 sites of the promoter); 2) lacZ reporter gene, expression of which is controlled by pSYN; and, 3) the tested DNA binding protein under regulation of the arabinose inducible (pBAD) promoter. The principle of our device is that the binding of a DNA binding protein to its specific target sequence in pSYN promoter would result in decreased basal activity of this promoter. The activity of pSYN was monitored measuring the activity of β-galactosidase as a reporter (see scheme below). E. coli bacteria, containing our device on a low copy plasmid were grown in the presence of increasing concentrations of arabinose to induce expression of the DNA binding protein. After overnight growth in Luria Bertani broth, β-galactosidase activity was measured and results expressed in Miller Units as described in protocols.

Results
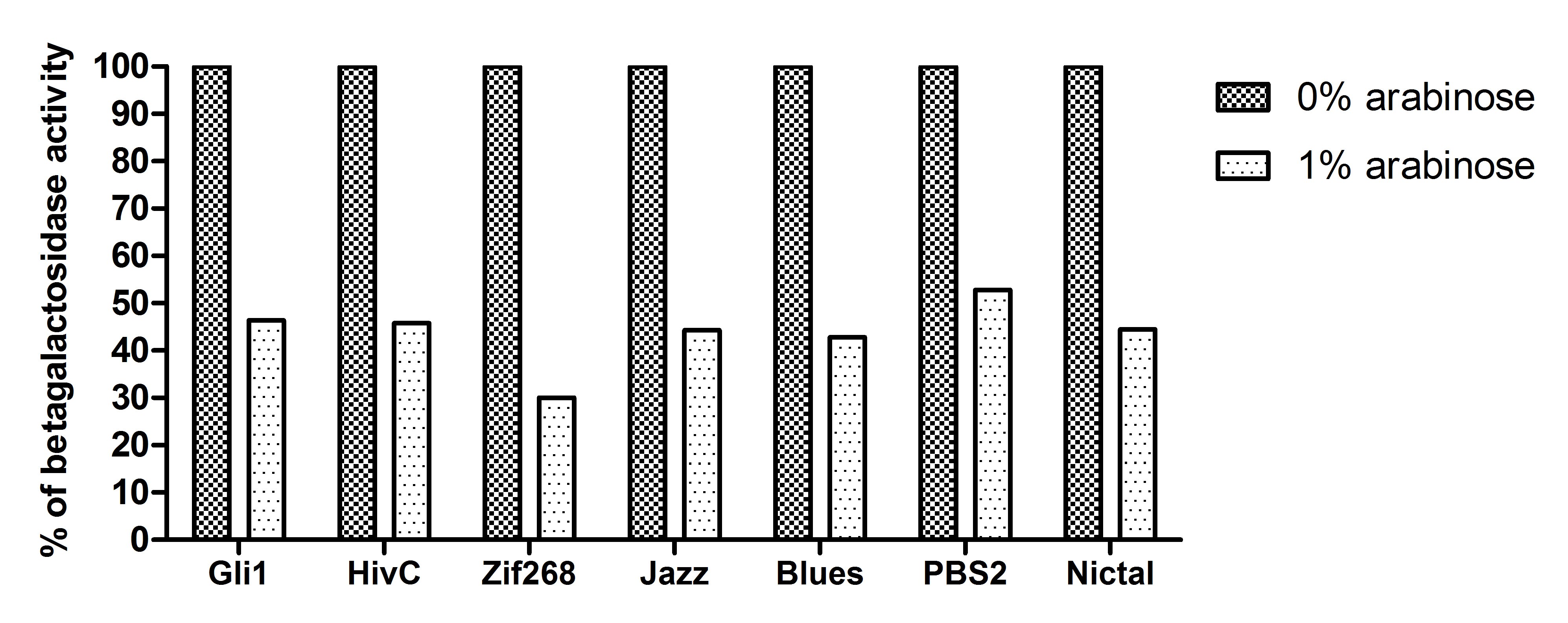
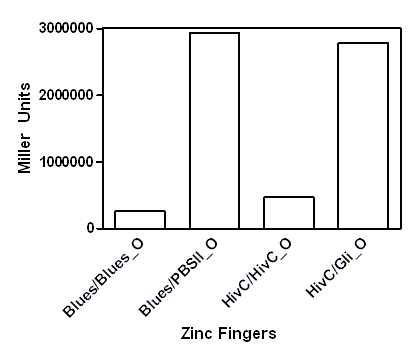
A representative of five experiments of our universal device for testing binding of DNA binding proteins is shown below. A full range of arabinose concentrations were tested, however, due to the ease of presentation only results +/- 1% arabinose are presented. To be able to compare β-galactosidase activities of each zinc finger construct among each other, results were expressed as % of 0% arabinose for each zinc finger construct. While 1% arabinose had no effect on β-galactosidase activity of E. coli carrying no plasmid construct (not shown), this concentration of arabinose resulted in ~60 % reduction of β-galactosidase activity in the E. coli containing our device expressing Gli1, HivC, Zif286, Jazz, Blues, PBSII zinc fingers or NicTAL DNA binding protein (see figure A, dotted bars). In contrast, β-galactosidase activity remained unchanged in the control devices, where pSYN promoter contained a binding site of a different zinc finger (compare bars for Blues/Blues_o and Blues/PBSII_O, where the first pair represents β-galactosidase activity of device expressing Blues and containing pSYN with the binding site for Blues, and a device expressing Blues and containing pSYN with the binding site for PBSII, respectively, see figure B). These results suggest that all zinc fingers used in our studies specifically bind to their corresponding DNA binding sites in vivo. Furthermore, we created a universal device that can be customized for testing of binding interactions of any DNA binding protein and its corresponding target DNA sequence.
 "
"