Team:Slovenia/PROJECT/biosynthesis
From 2010.igem.org
| Line 37: | Line 37: | ||
<p> </p> | <p> </p> | ||
<p> </p> | <p> </p> | ||
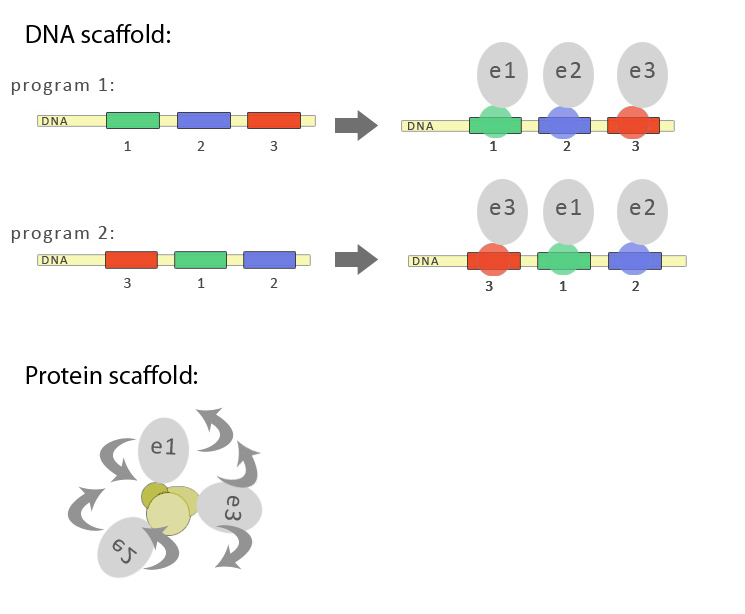
| - | [[Image:Slo_dna_scafold1_1.jpg | + | [[Image:Slo_dna_scafold1_1.jpg|frame|x650px|'''Schematic representation of advantages of DNA-guided biosynthetic scaffold. DNA imparts the linear order, while protein/polypeptide based scaffold predominantly clusters the biosynthetic enzymes without of particular order. |
''']] | ''']] | ||
<p> </p> | <p> </p> | ||
Revision as of 08:05, 27 October 2010
Introduction
Biosynthesis is an enzyme catalyzed process, occurring in living cells, by which simple substrate molecules are converted into more complex products. The process often consists of several steps, in which the product of one step is used as a substrate for the following step. In synthetic biology, research and engineering of biosynthetic pathways gains more and more attention every year. One of the first great stories in the field of synthetic biology was engineering the artificial biosynthetic pathway for antimalarial drug artemisinic acid production in Saccharomyces cerevisae yeast. Production of artemisinic acid in genetically modified yeasts was achieved modulating regulation of specific mevalonate pathway genes and by the introduction of genes for the biosynthetic pathway from Artemisia annua plant to yeast. Another great examples of biosynthetic pathway engineering are production of fatty esters (biodiesel), fatty alcohols, and waxes by genetically modified Escherichia coli. Genes from different organisms were combined into completely new pathway which was introduced to Escherichia coli to produce useful fuel directly from plant biomass.
For industrial applications biosynthetic pathways composed of several enzymes should be engineered in a way to achieve high yield of the desired biosynthetic products. Various strategies for optimization have been undertaken so far, such as:
- Increasing the pool of available substrate and/or overexpression of the enzymes of the limiting biosynthetic steps,
- Introducing heterologous enzymes with preferred kinetic characteristics,
- Blocking branching of biosynthetic pathway,
- Compartmentalizing of biosynthetic pathways by directing enzymes of a particular biosynthetic pathway to a specific cell compartments or artificially made compartments (e.g. metabolosomes),
- Increasing the proximity of enzymes by assembling metabolic pathways on a protein scaffold.
Scaffold-assisted biosynthetic pathway
The last approach is in some way similar to solutions that nature has already evolved. In some natural biosynthetic pathways enzymes form larger complexes, which results in faster transport of intermediates from one enzyme to another. This strategy enables an organism to produce higher amounts of the final product with lower metabolic burden. Unstable and toxic intermediates can be protected from decay or can be neutralized, since they are immediately used by the next enzyme in the pathway. Experimental data has already shown on a case of resveratrol biosynthesis (Zhang et al., 2006) where fusing two enzymes together improves the efficiency of this particular biosynthetic pathway. However, fusing more than two enzymes may prove to be difficult on account of maintaining the functionality of the fusion protein, which is the reason that scaffolding is a better approach. A protein scaffold has already been used to improve the mevalonate pathway yield (Dueber et al. 2009). While there are many advantages of protein-based scaffold, this approach has some disadvantages. Three dimensional arrangement of polypeptides is unpredictable due to the flexibility of and between dimerization domains as represented in the scheme. Additionally each protein dimerization domain has different conditions under which it folds and forms the functional interaction. And perhaps the most important argument, there is a limited number of available weel-behaved protein dimerization domains available, while the biosynthetic pathways may comprise ten or more enzymes and synthetic bioengineers will want to engineer several pathways simultaneously.
 "
"