Team:SDU-Denmark/project-r
From 2010.igem.org
(Difference between revisions)
(→Results) |
(→Results) |
||
| Line 16: | Line 16: | ||
[[Image:Team-SDU-Denmark-DH5alpha.JPG|250px]] | [[Image:Team-SDU-Denmark-DH5alpha.JPG|250px]] | ||
<br> | <br> | ||
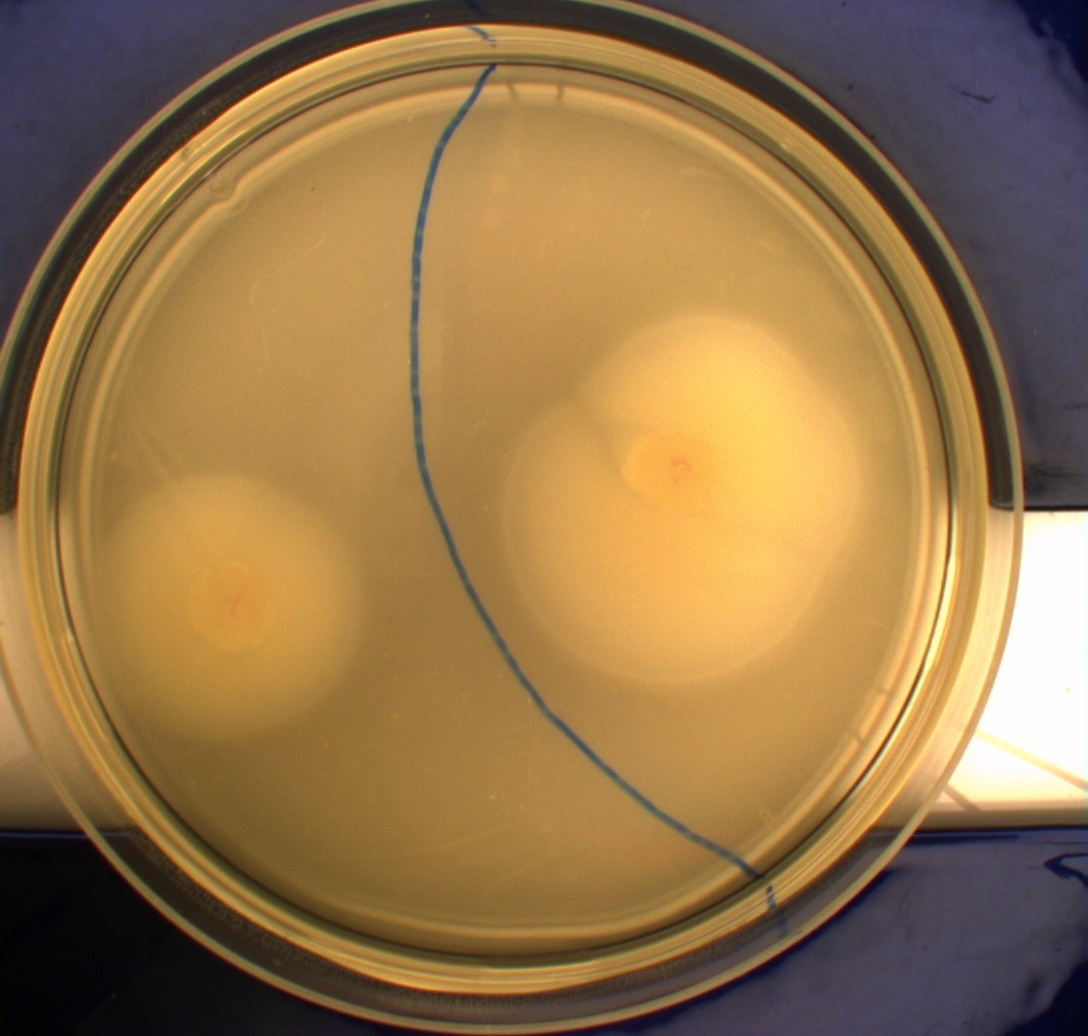
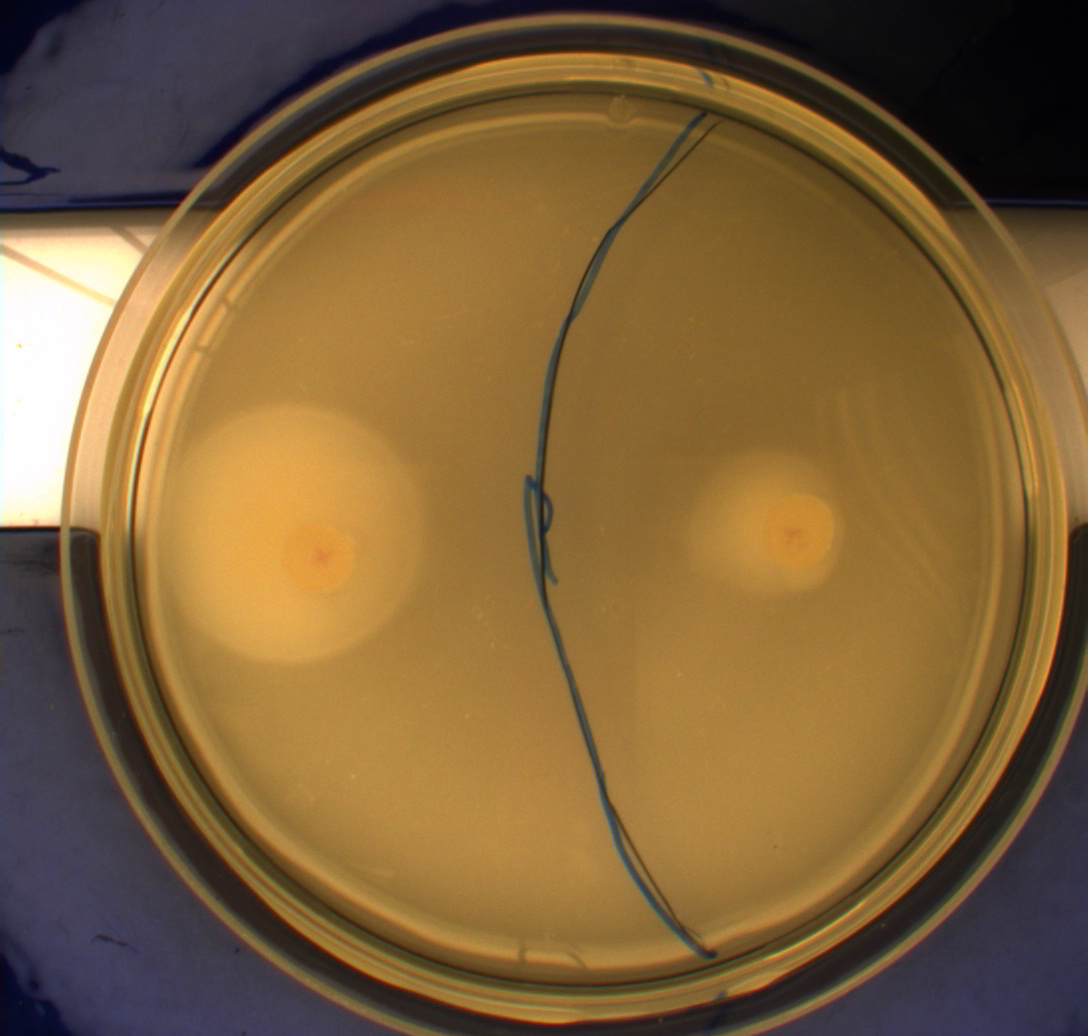
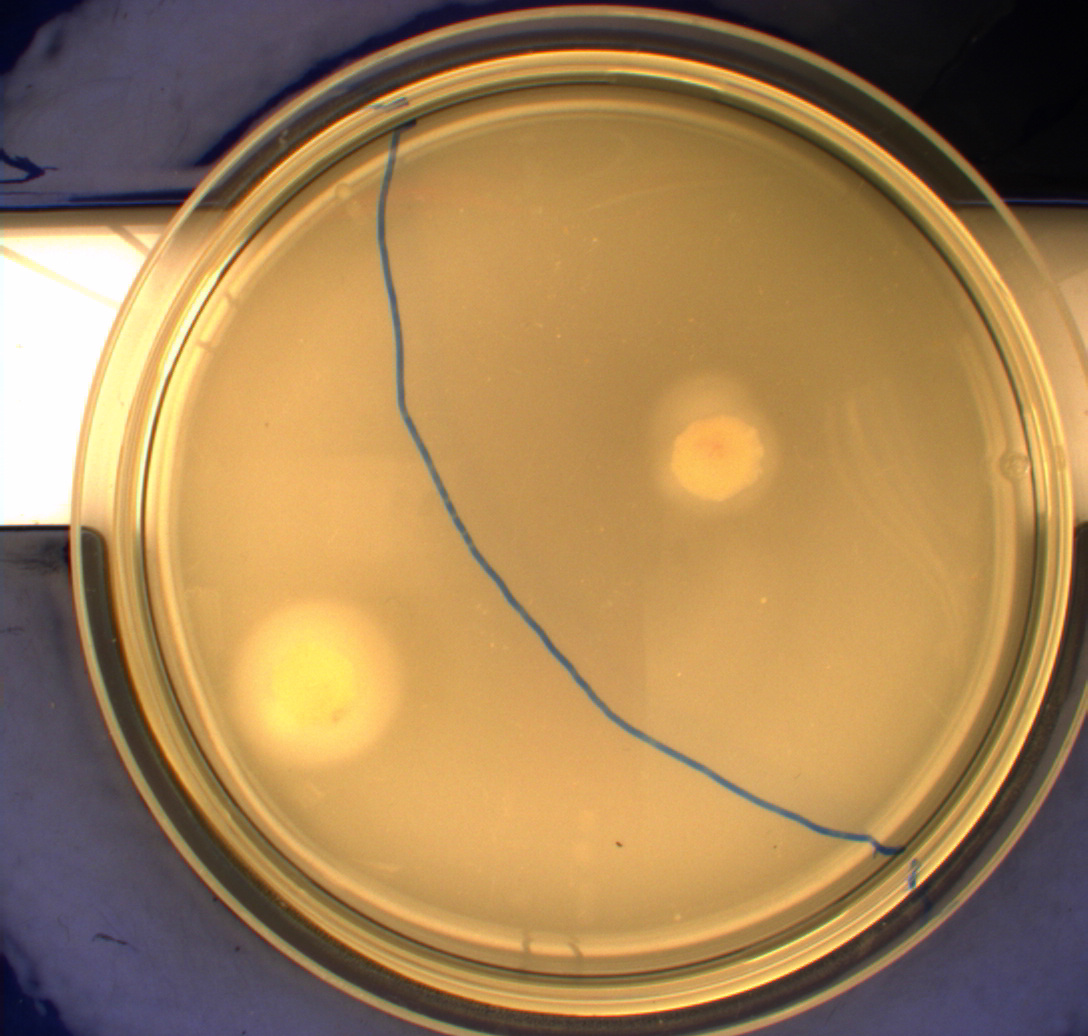
| - | Interpreting these results is not totally obvious since the effect of different motility patterns can be counter intuitive in semisolid agar. An explanation as to how different motility patterns show up on semisolid agar plates is explained by Englert et al. in the book "Microfluidic techniques for the analysis of bacterial chemotaxis": Polymerized agar consists of chains of extended polymers permeated by water-filled channels. At low agar concentrations (0.25– | + | Interpreting these results is not totally obvious since the effect of different motility patterns can be counter intuitive in semisolid agar. An explanation as to how different motility patterns show up on semisolid agar plates is explained by Englert et al. in the book "Microfluidic techniques for the analysis of bacterial chemotaxis":<br><br> |
| - | 0.4% | + | |
| - | cells that only swim smoothly get trapped cul de sacs in the agar matrix (16), and their colonies do not expand significantly faster than those of nonmotile cells. | + | ''Polymerized agar consists of chains of extended polymers permeated by water-filled channels. At low agar concentrations (0.25– 0.4% semisolid agar) the channels are sufficiently large that the bacteria can swim through them. In the absence of chemoeffectors, cells conduct a 3D random walk through the agar matrix much as they would in liquid and spread out randomly from the point of inoculation. Since the cells are typically growing, the result is an expanding colony that forms within the agar. This “pseudotaxis” occurs in the absence of any tactic behavior. Mutant |
| + | cells that only swim smoothly get trapped cul de sacs in the agar matrix (16), and their colonies do not expand significantly faster than those of nonmotile cells.'' <br><br> | ||
| + | |||
| + | This means that if the photosensor leads to a reduced tumbling rate when exposed to blue light, it should look a lot like the colony of DH5alpha whichw as exposed to blue light. This is exactly the case which proves that the photosensor has an effect on the tumbling rate of the bacteria. The photosensor colony in the dark behaved like the Mg1655 colony in the dark and spread out. This is normal as cells that do not tend to either tumble or run a lot, will spread the most. <br><br> | ||
Revision as of 13:38, 13 October 2010
 "
"