Team:Paris Liliane Bettencourt
From 2010.igem.org
Aleksandra (Talk | contribs) |
Aleksandra (Talk | contribs) |
||
| Line 18: | Line 18: | ||
<br>Counting is the action of finding the number of elements in a set. Counting is a basic operation in electronic circuitry accomplished by the use of flip-flops, which are a type of digital device that has two stable states and can act as a single “bit” of memory for a counter. | <br>Counting is the action of finding the number of elements in a set. Counting is a basic operation in electronic circuitry accomplished by the use of flip-flops, which are a type of digital device that has two stable states and can act as a single “bit” of memory for a counter. | ||
| - | + | ||
| - | + | <br>Early efforts by synthetic biologists have shown that implementing this type of counter in cells is not easy. Since components in a cell are not strictly sequestered in the manner of electronic circuits, and since the cell signalling channels often interfere with each other in ways that are difficult to predict, digital methods seem like a problematic way to count in cells. | |
| - | <br>The method of counting in our project is capable both of true counting and of threshold detection, and is more similar to the mechanical counters invented in the late 19th century than to any digital circuit. | + | |
| - | <br> | + | <br>When looking to nature for inspiration, one finds mostly circuits that don’t actually count in a strict sense, but instead act as “threshold detectors” that cause something to happen after a certain threshold has been reached. Cell aggregation, telomere length regulation, and quorum sensing all fall under this category of device. |
| - | + | ||
| - | + | <br>The first method of counting in our project is capable both of true counting and of threshold detection, and is more similar to the mechanical counters invented in the late 19th century than to any digital circuit. | |
| + | |||
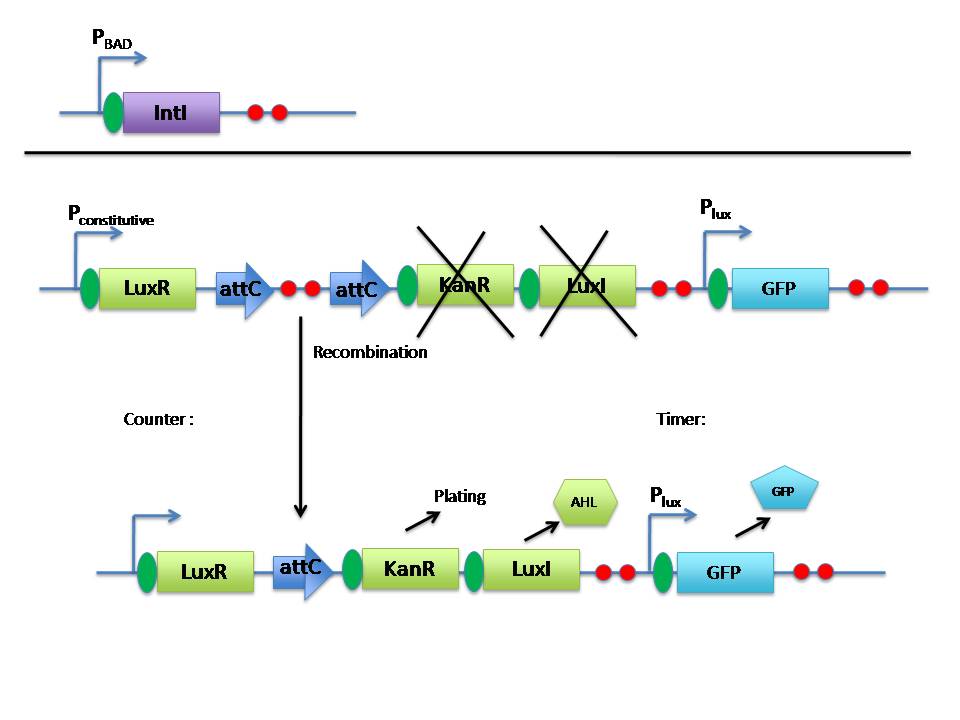
| + | <br>We have designed a “rotary counter” that counts events within a single cell. Each time the cell detects a pulse of input, it performs an excision and an integration, activating a reporter. The number of integrated sequences corresponds in a one-to-one fashion with the number of inductions, serving as an accurate counter. The key to our systems accuracy is a specially designed system that preferentially integrates sequences only to sites directly downstream on the genome, allowing us to integrate in a sequential “rotary” fashion and avoid skips in the count. Our systems output can be read manually by PCR, or by adding reporter genes directly after any of the integration sites. | ||
| + | |||
| + | <br>For example, one could integrate a gene that produces a protein of interest to the third recombination site, and a lysis gene to the fifth site. In this way, the system can act as a timer as well as a counter, producing protein for a period of time, and then releasing it to the media at another time. Since not all the cells in the population will count correctly, there is a fail-safe mechanism that kills any cell that is “off-beat” by using a small intra-cellular toxin. | ||
| + | |||
| + | <br>Our second experiment is based on a wholly different way to count. By relying on the statistical ability to predict the behavior of a rare event occuring in a large population, and by exploiting cells ability to talk to each-other, we are creating a “population counter.” Each cell in our population harbors a construct that, after induction, has a small chance of starting the expression of an antibiotic-resistance gene. The proportion of resistant cells in the population reflects the number of induction pulses in a highly predictable fashion, thus allowing another type of counter. | ||
| + | |||
| + | <br>This counter, like the first one, can also be used in “timer” mode. Timer functionality is achieved by co-expressing with the antibiotic gene LuxI, under a promoter of our choice. When the media concentration of AHL exceeds a threshold (in part determined by the promoter we choose) YFG is expressed. To be able to count correctly, this system requires careful control of the media, so we have designed a microfluidic device to test this system. | ||
[[Image:populationcounter1.jpg]] | [[Image:populationcounter1.jpg]] | ||
Revision as of 21:37, 16 July 2010
iGEM > Paris > Home > Synopsis from old template...
Memo-Cell
Abstract
Counting is the action of finding the number of elements in a set. Counting is a basic operation in electronic circuitry accomplished by the use of flip-flops, which are a type of digital device that has two stable states and can act as a single “bit” of memory for a counter.
Early efforts by synthetic biologists have shown that implementing this type of counter in cells is not easy. Since components in a cell are not strictly sequestered in the manner of electronic circuits, and since the cell signalling channels often interfere with each other in ways that are difficult to predict, digital methods seem like a problematic way to count in cells.
When looking to nature for inspiration, one finds mostly circuits that don’t actually count in a strict sense, but instead act as “threshold detectors” that cause something to happen after a certain threshold has been reached. Cell aggregation, telomere length regulation, and quorum sensing all fall under this category of device.
The first method of counting in our project is capable both of true counting and of threshold detection, and is more similar to the mechanical counters invented in the late 19th century than to any digital circuit.
We have designed a “rotary counter” that counts events within a single cell. Each time the cell detects a pulse of input, it performs an excision and an integration, activating a reporter. The number of integrated sequences corresponds in a one-to-one fashion with the number of inductions, serving as an accurate counter. The key to our systems accuracy is a specially designed system that preferentially integrates sequences only to sites directly downstream on the genome, allowing us to integrate in a sequential “rotary” fashion and avoid skips in the count. Our systems output can be read manually by PCR, or by adding reporter genes directly after any of the integration sites.
For example, one could integrate a gene that produces a protein of interest to the third recombination site, and a lysis gene to the fifth site. In this way, the system can act as a timer as well as a counter, producing protein for a period of time, and then releasing it to the media at another time. Since not all the cells in the population will count correctly, there is a fail-safe mechanism that kills any cell that is “off-beat” by using a small intra-cellular toxin.
Our second experiment is based on a wholly different way to count. By relying on the statistical ability to predict the behavior of a rare event occuring in a large population, and by exploiting cells ability to talk to each-other, we are creating a “population counter.” Each cell in our population harbors a construct that, after induction, has a small chance of starting the expression of an antibiotic-resistance gene. The proportion of resistant cells in the population reflects the number of induction pulses in a highly predictable fashion, thus allowing another type of counter.
This counter, like the first one, can also be used in “timer” mode. Timer functionality is achieved by co-expressing with the antibiotic gene LuxI, under a promoter of our choice. When the media concentration of AHL exceeds a threshold (in part determined by the promoter we choose) YFG is expressed. To be able to count correctly, this system requires careful control of the media, so we have designed a microfluidic device to test this system.
 "
"