Team:Newcastle/Filamentous Cells
From 2010.igem.org

| |||||||||||||
| |||||||||||||
Contents |
Filamentous cell formation by overexpression of yneA
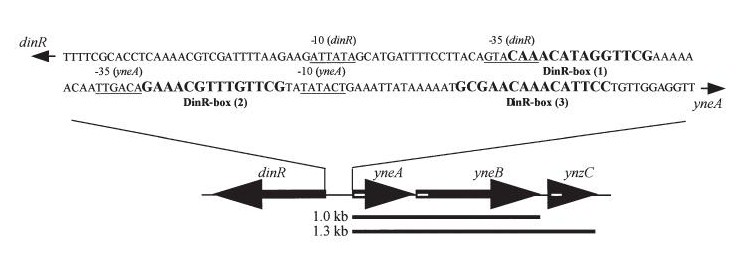
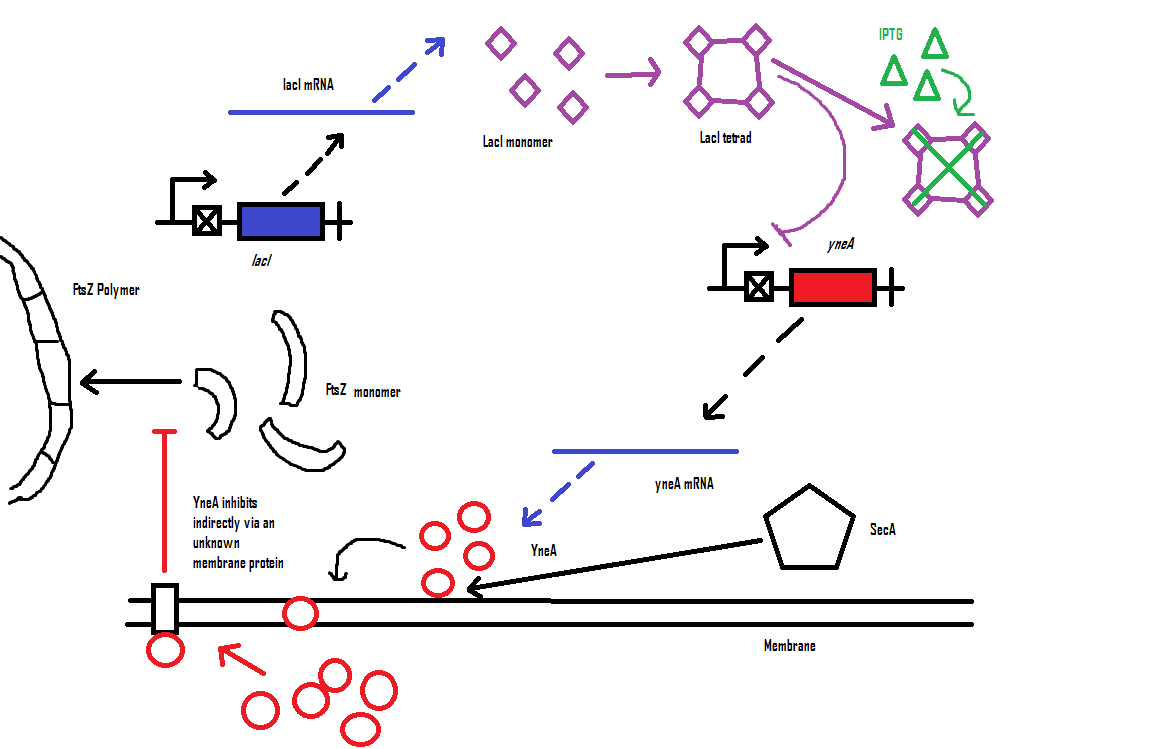
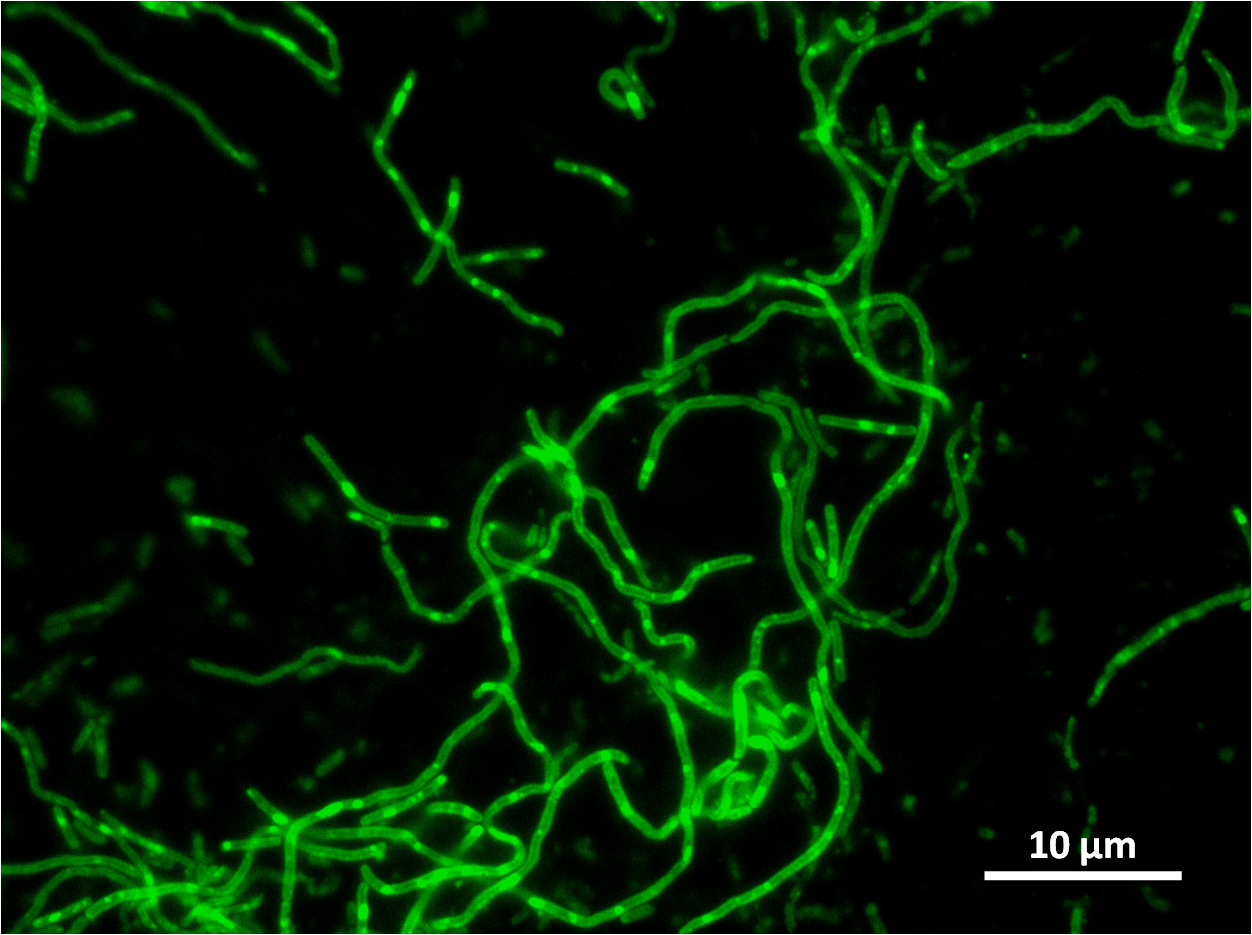
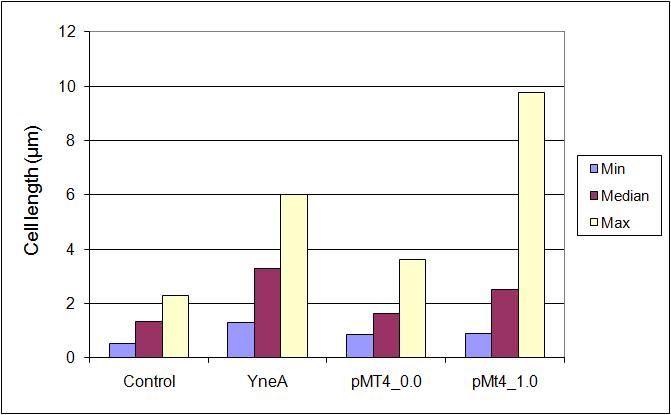
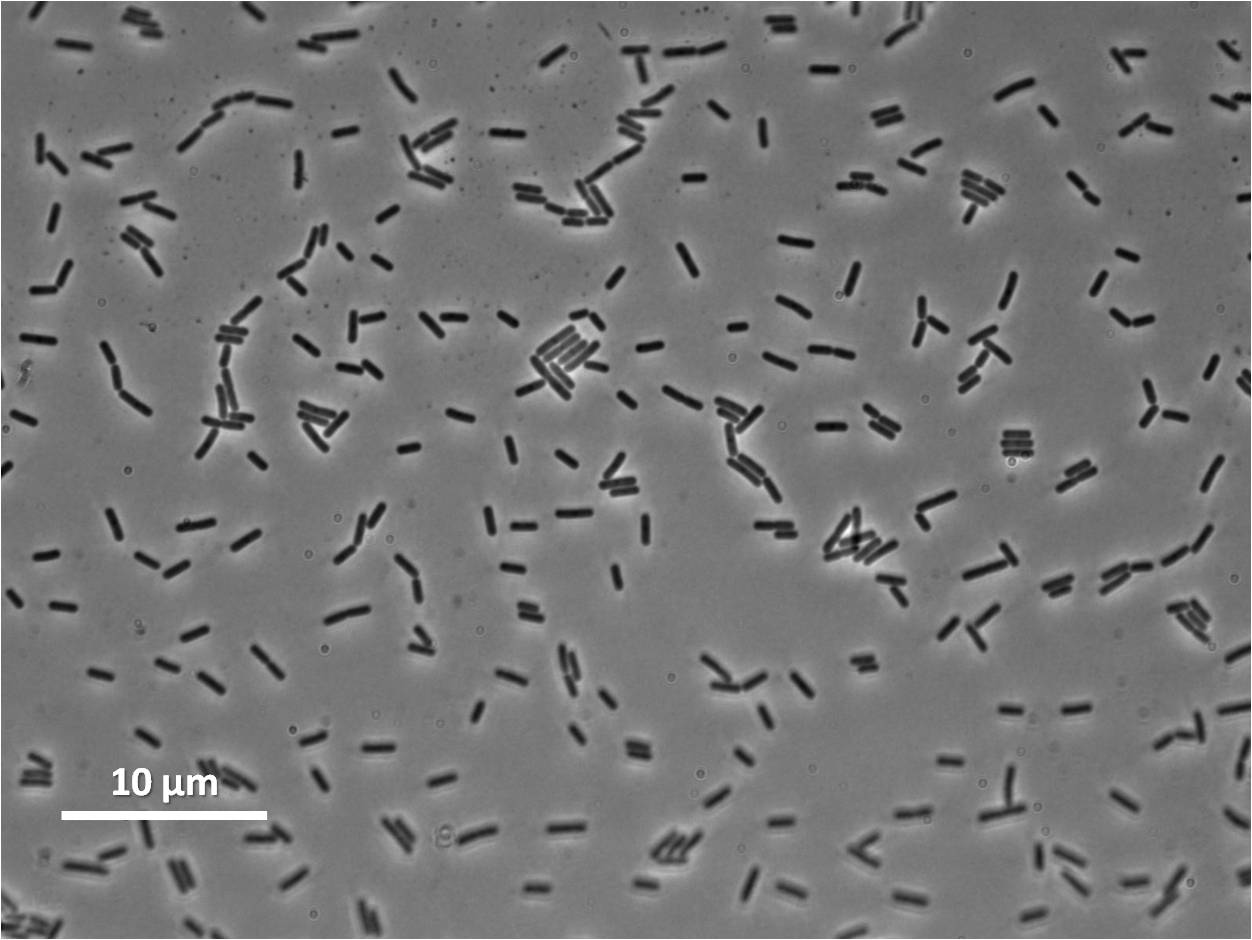
Bacillus subtilis in response to stress such as DNA damage stops the cells from dividing. This is a part of the SOS response initiated by the accumulation of single stranded DNA from DNA damage or stalled replication. Two proteins are vital for this response: RecA and LexA. RecA forms filaments on ssDNA and promotes the autocleavage of LexA. LexA usually represses the SOS operon. dinR is homologous to lexA in E. coli and is transcribed in the opposite direction of yneA. YneA stops the formation of FtsZ ring indirectly. When FtsZ forms a 30 subunit ring at the midpoint of the cell, it will contract and cause cell division. By expressing YneA and inhibiting FtsZ ring formation, the cells will grow filamentous. By inhibiting cell division, YneA allows the DNA damage genes to repair the DNA damage before continuing with the cell division cycle. It is hypothesized that YneA acts through an unknown transmembrane protein to inhibit FtsZ ring formation; we call this/these unknown components “Blackbox proteins”. As the evidence shows expression of yneA leads to filamentation.
Research
SOS response is believe to be a universal bacteria phenomenon first studied in E.coli -lexA, recA
In Bacillus subtillis (gram positive) dinR protein is homologous to lexA (Repressor of din-damage inducible genes). din genes include uvrA, uvrB, dinB, dinC dinR and recA. DNA damage inhibits cell division.
| Wild type Bacillus subtilis | dinRKO Mutant |
|---|---|
| 
|
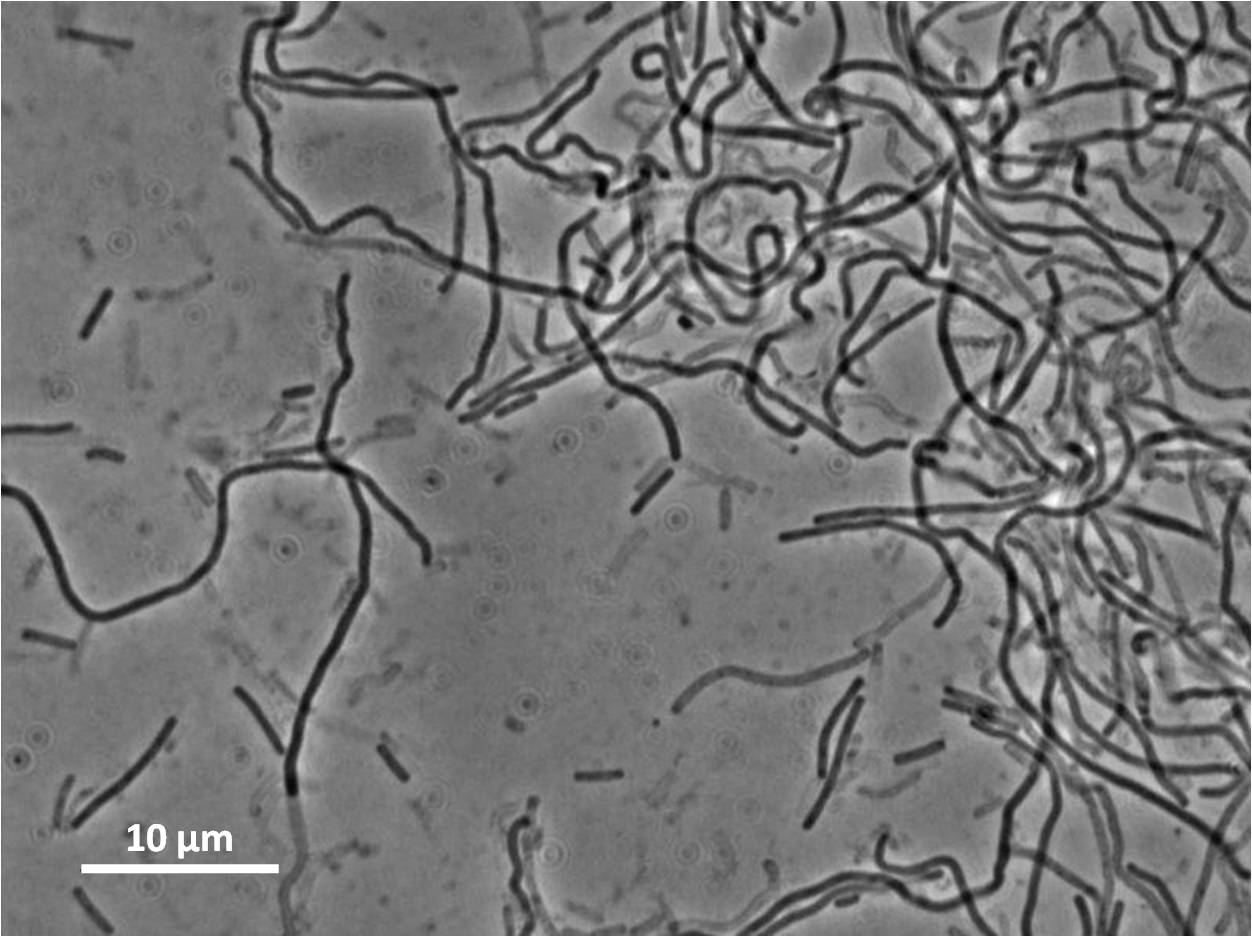
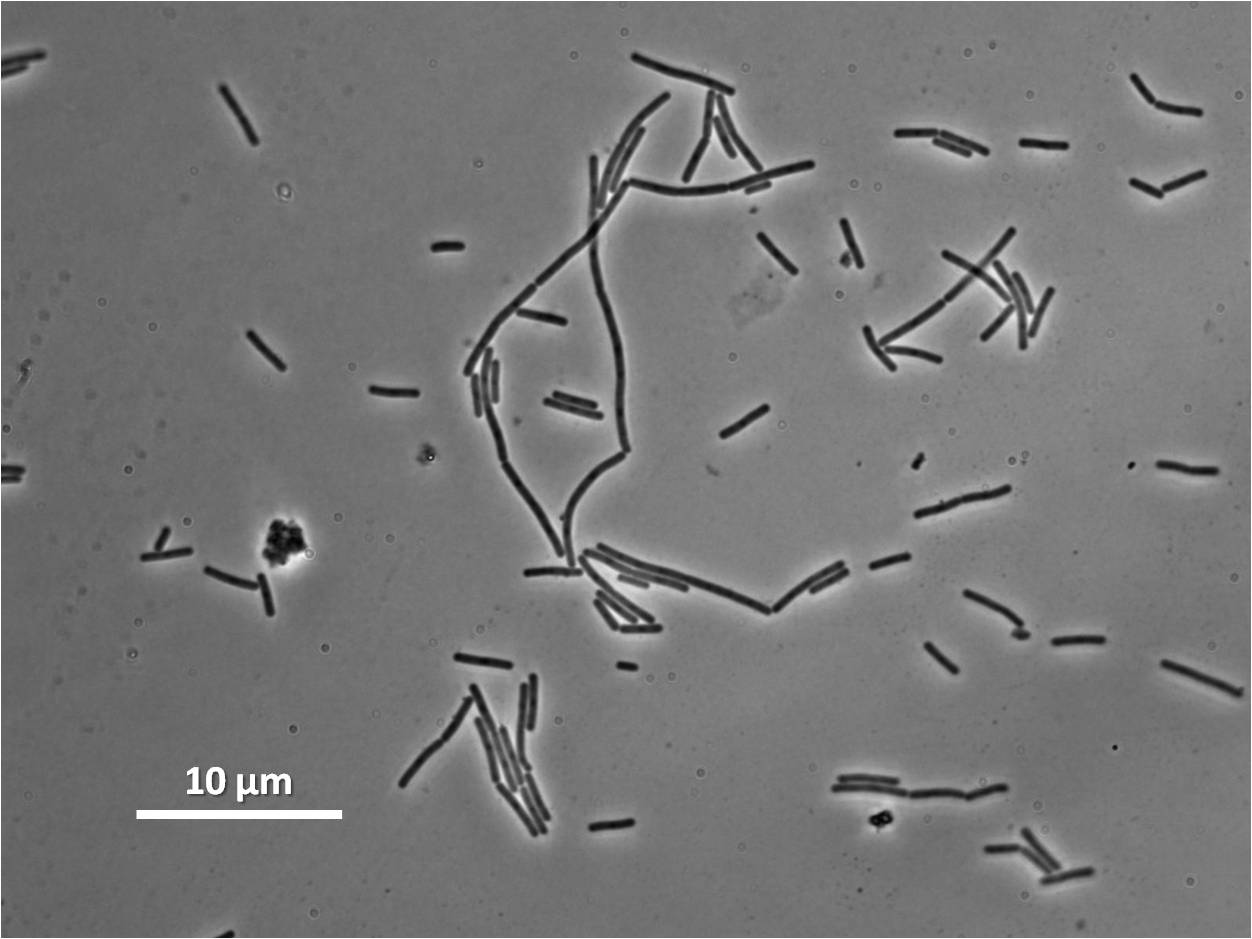
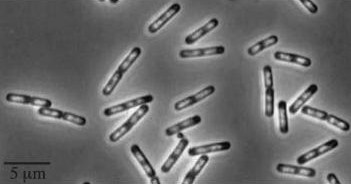
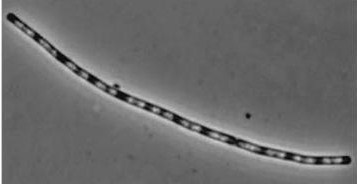
Figure1: The images above show Bacillus subtilis Wild type and dinRKO mutant, and the change in cell length. dinR KO mutant over expresses the divergent (opposite direction) transcript for YneA, YneB and YnzC. These genes form the SOS regulon (recA independent SOS response).
Figure2:The diagram above shows the Coding region for dinR and yneA showing divergent expression.
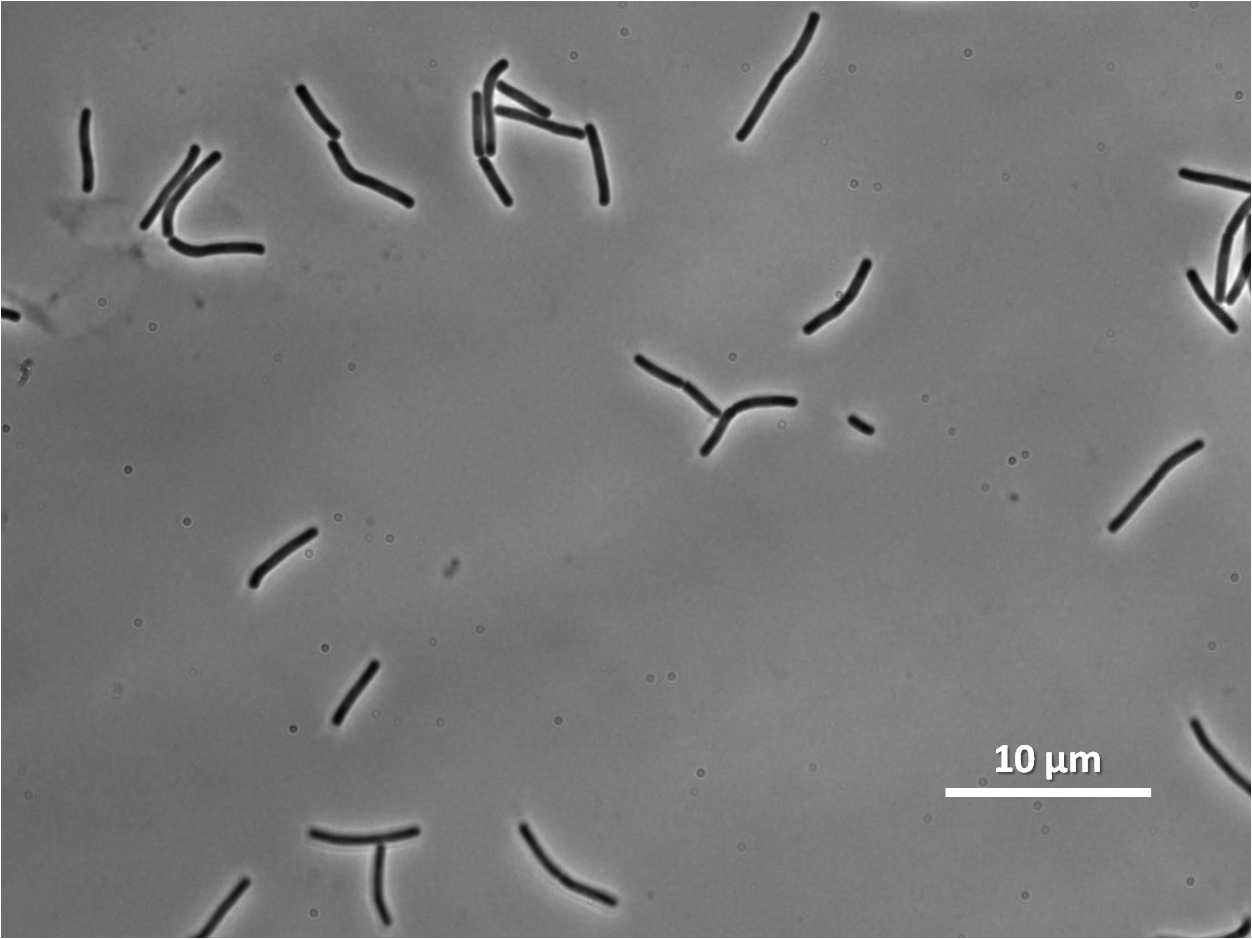
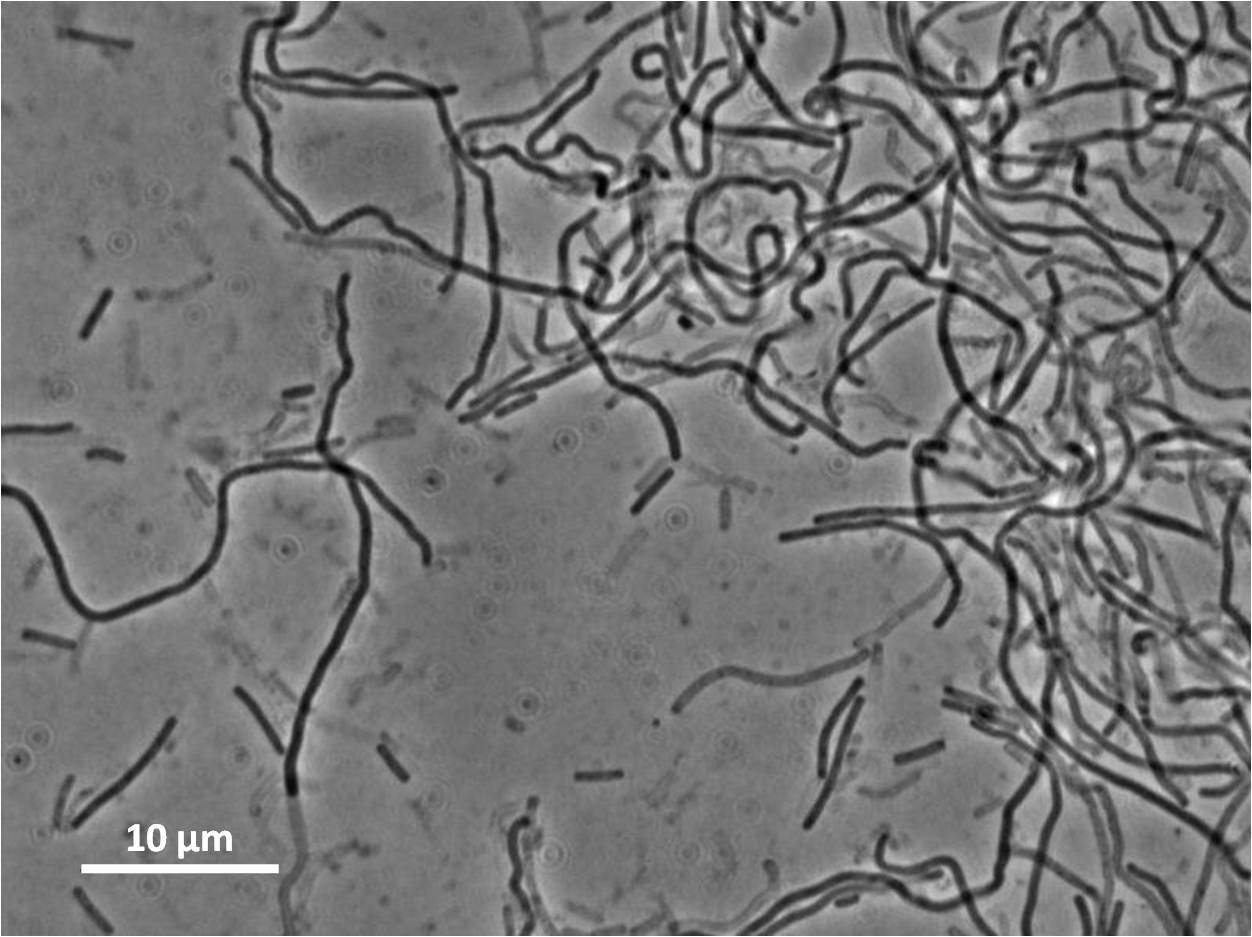
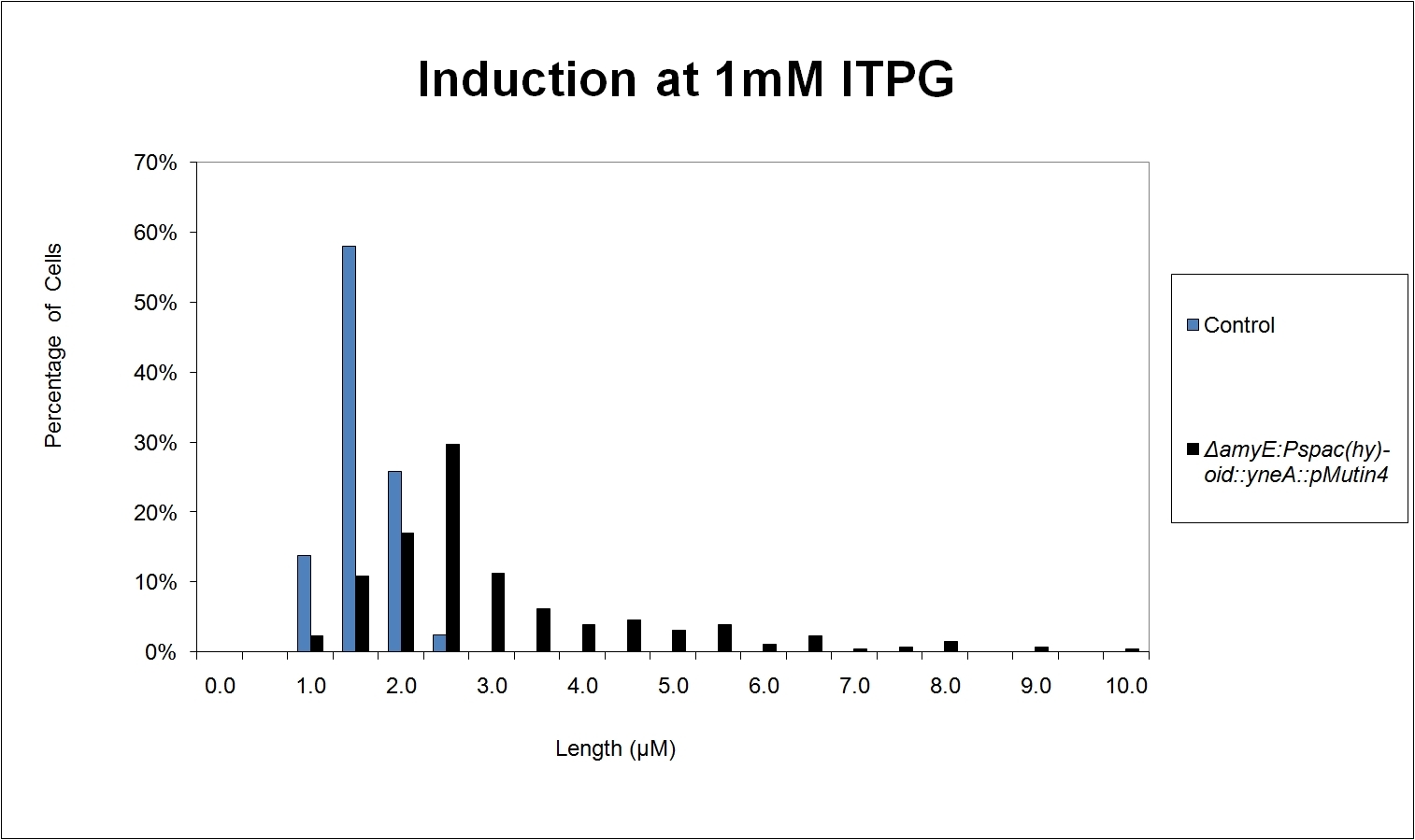
Expression of YneA from IPTG controlled promoter in wildtype leads to elongation. Disruption of YneA in SOS response leads to reduced elongation. Altering YneB and YnzC expression does not affect cell morphology.
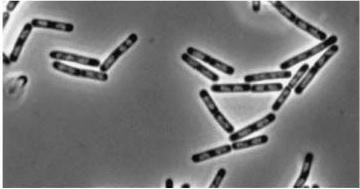
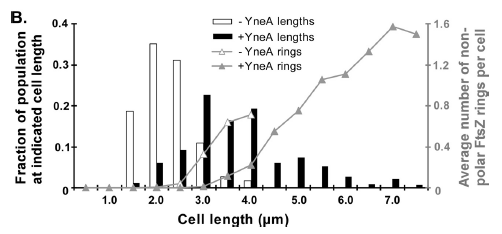
| 
|
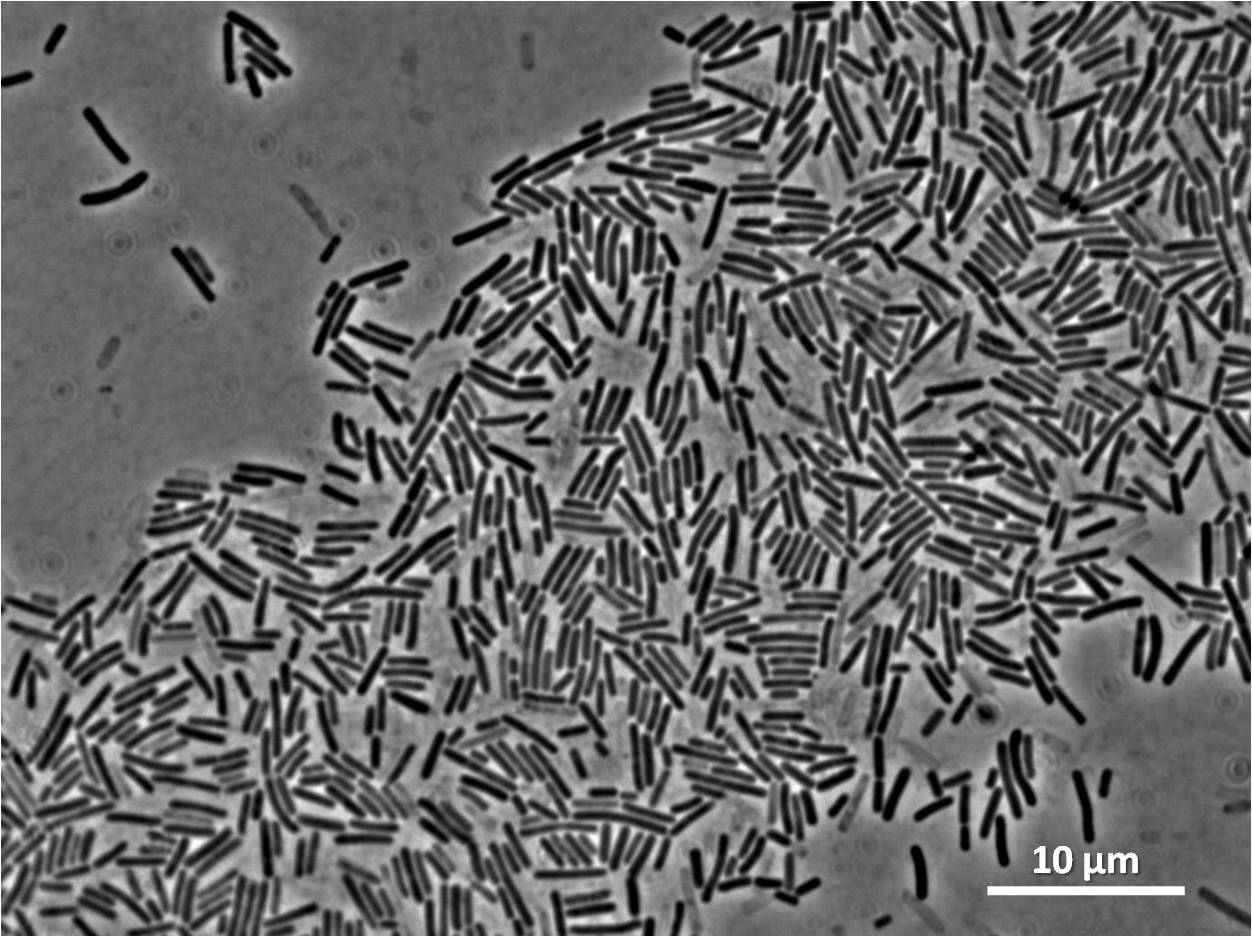
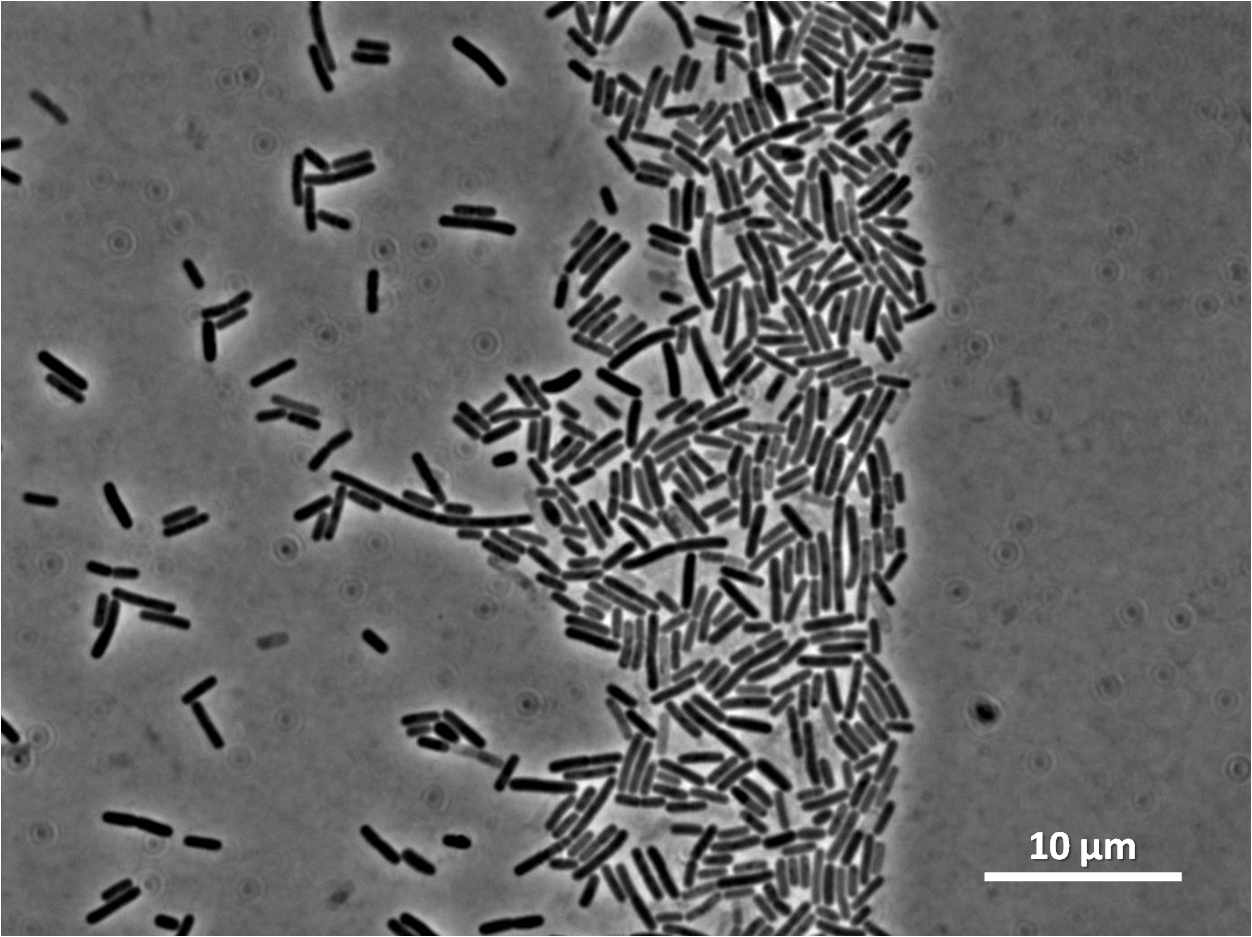
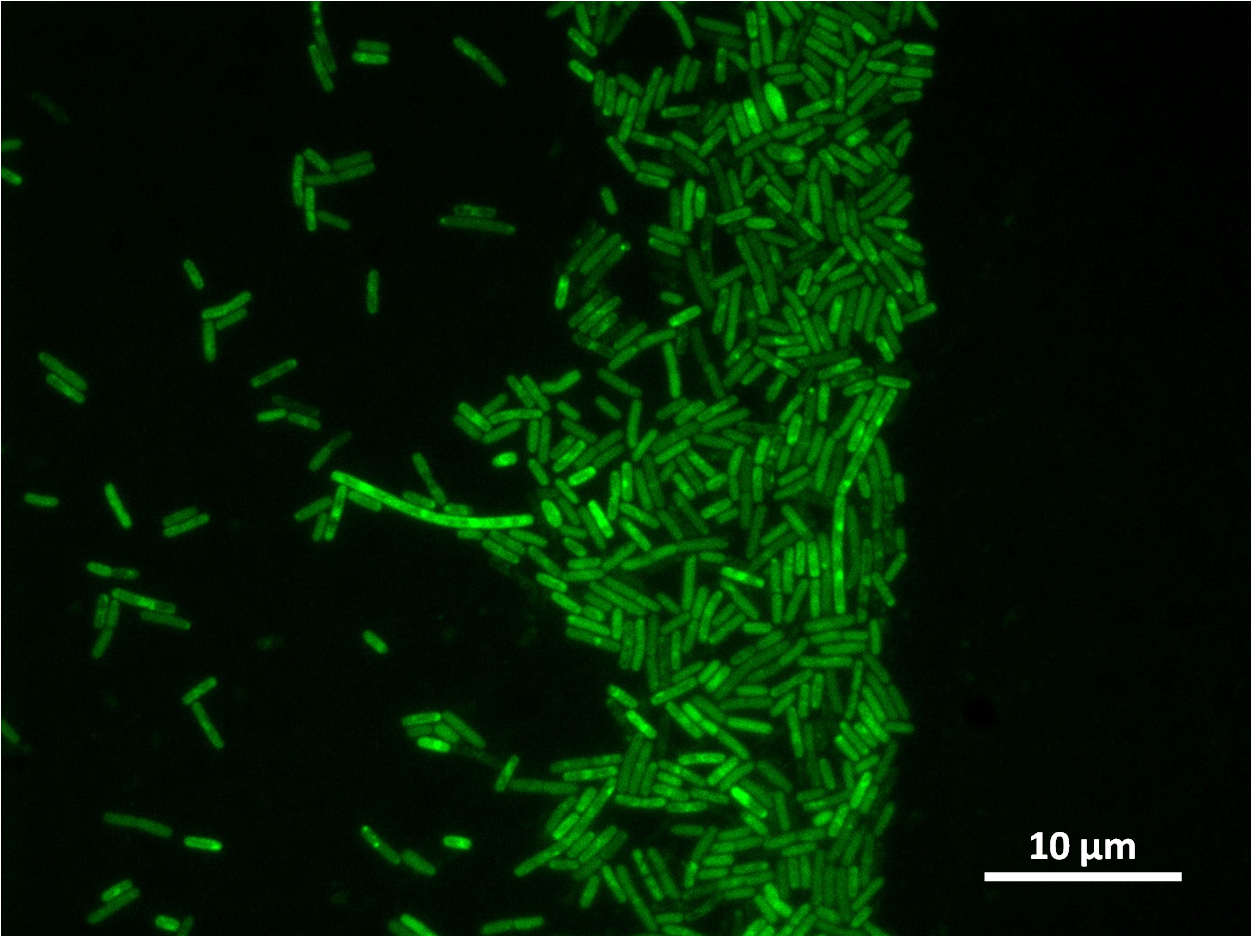
Figure3(above left): Shows the double mutant dinR overexpression cancels out the filament formation via over expression of yneA.
Figure4(above right):This graph shows the correllation between reduced FtsZ ring formation, increased cell length and overexpression of yneA.
YneA protein required to suppress cell division and not chromosome replication or segregation.
FtsZ is important for bacterial cell division forming a ring structure at the division site by polymerising assembling other proteins necessary for division at the site.
FtsZ localises to the cell division cycle unless dinR is disrupted or YneA is being induced. YneA suppresses FtsZ ring formation which is proven by 2 hybrid protein association test.
YneA expression by the inactivation of dinR by RecA is important.
Coding Sequence
Sequence of YneA: http://www.ncbi.nlm.nih.gov/nuccore/NC_000964.3?from=1918391&to=1918738&report=graph&content=5
yneA Biobrick
Biochemical Network
Computational Models
| Model1: | |
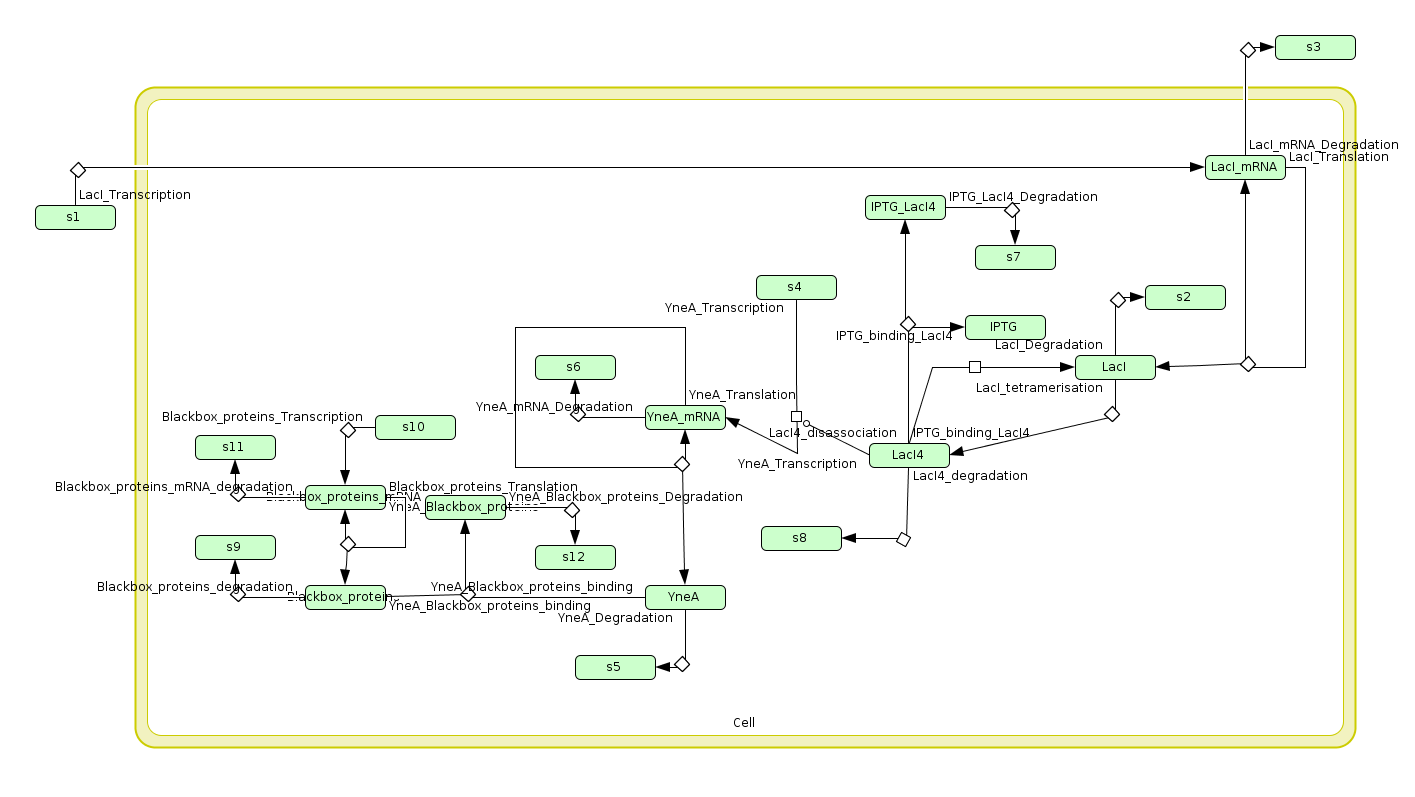
| This model was written in SBML and simulated in cell designer,it shows the species and compartment involved in our yneA system. |
| Model2: | |
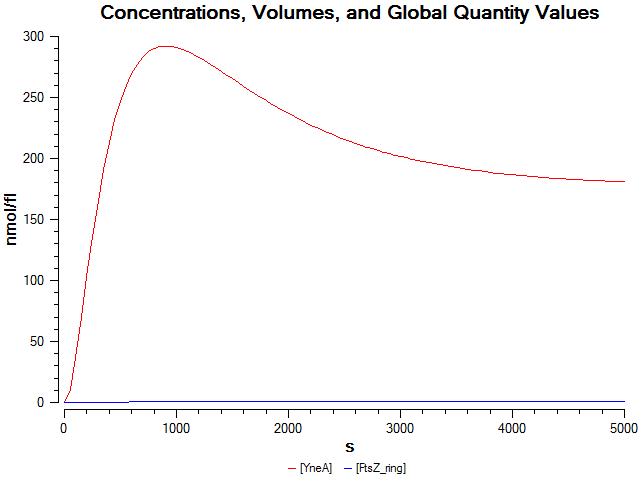
| We wrote a computational model of our filamentous cell system in SBML and simulated it in COPASI. The graph below shows that FtsZ ring formation is low when yneA is overexpressed. |
Cloning strategy
Media:yneA cloning strategy.pdf
Characterisation
Testing and Characterisation
Selection for integration
To select for integration of the plasmid into the chromosome, B. subtilis will be tested for the ability to hydrolyse starch. Integration of the BioBrick will be done by homologous recombination at the amyE, therefore destroying endogenous expression of amylase. Colonies that are not able to break down starch on agar plate will be selected and cultured for further test. Colonies that do not contain the integrated BioBrick will be able to hydrolyse starch, therefore forming a white halo around the colony as iodine interacts with starch to form blue colour.
yneA - with insert with IPTG – cell growth /yneA fluorescence (gfp transcription follows yneA If the bricks work- cut yneA (from vector it arrived in) with EcoR1 and Spe1 add double terminator to make it biobrick compatible ligate, run gel, gel extraction/purify. Cut with Pst1 and EcoR1 and ligate into Biobrick compatible vector to send to registry.
Lab work and Results
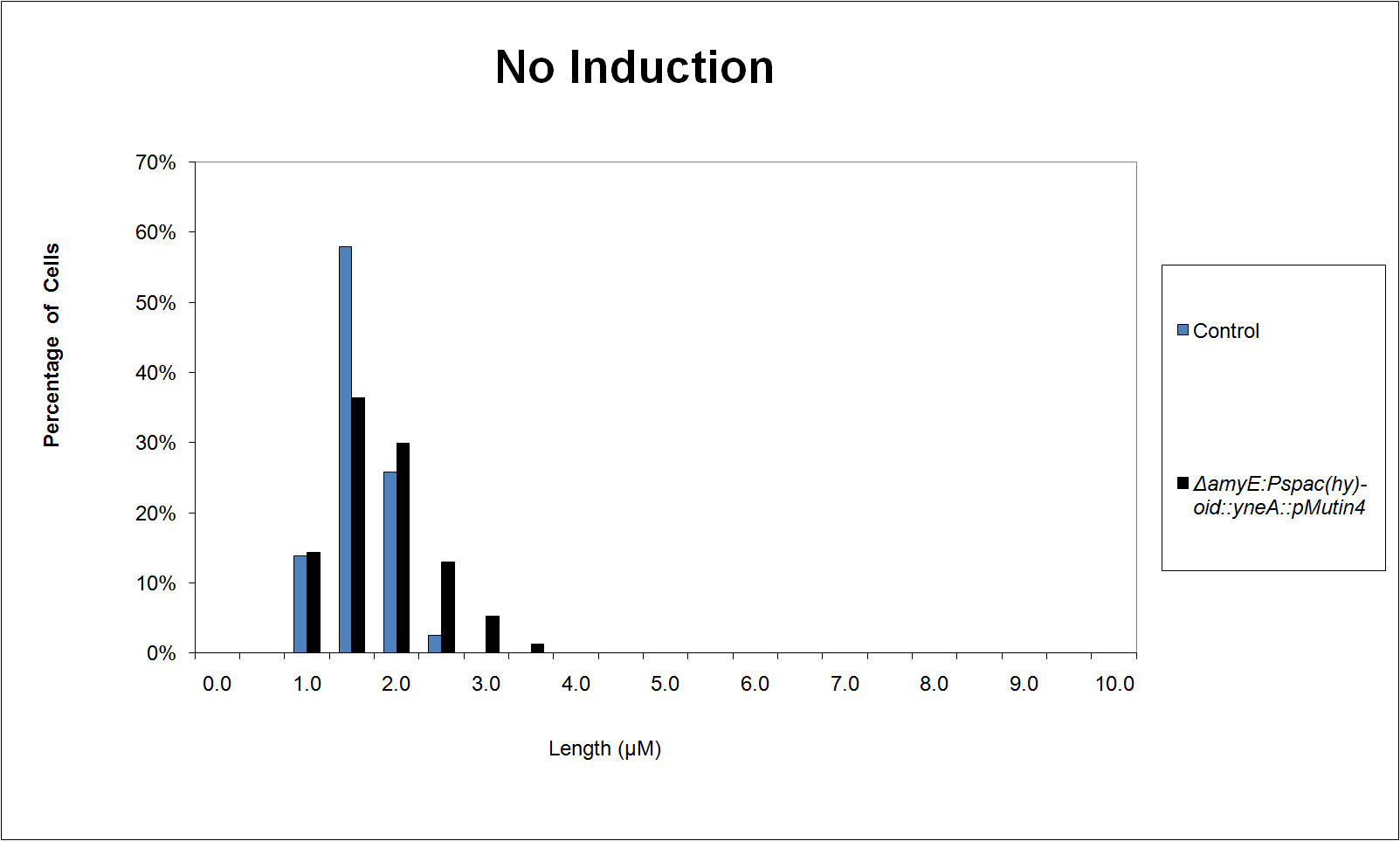
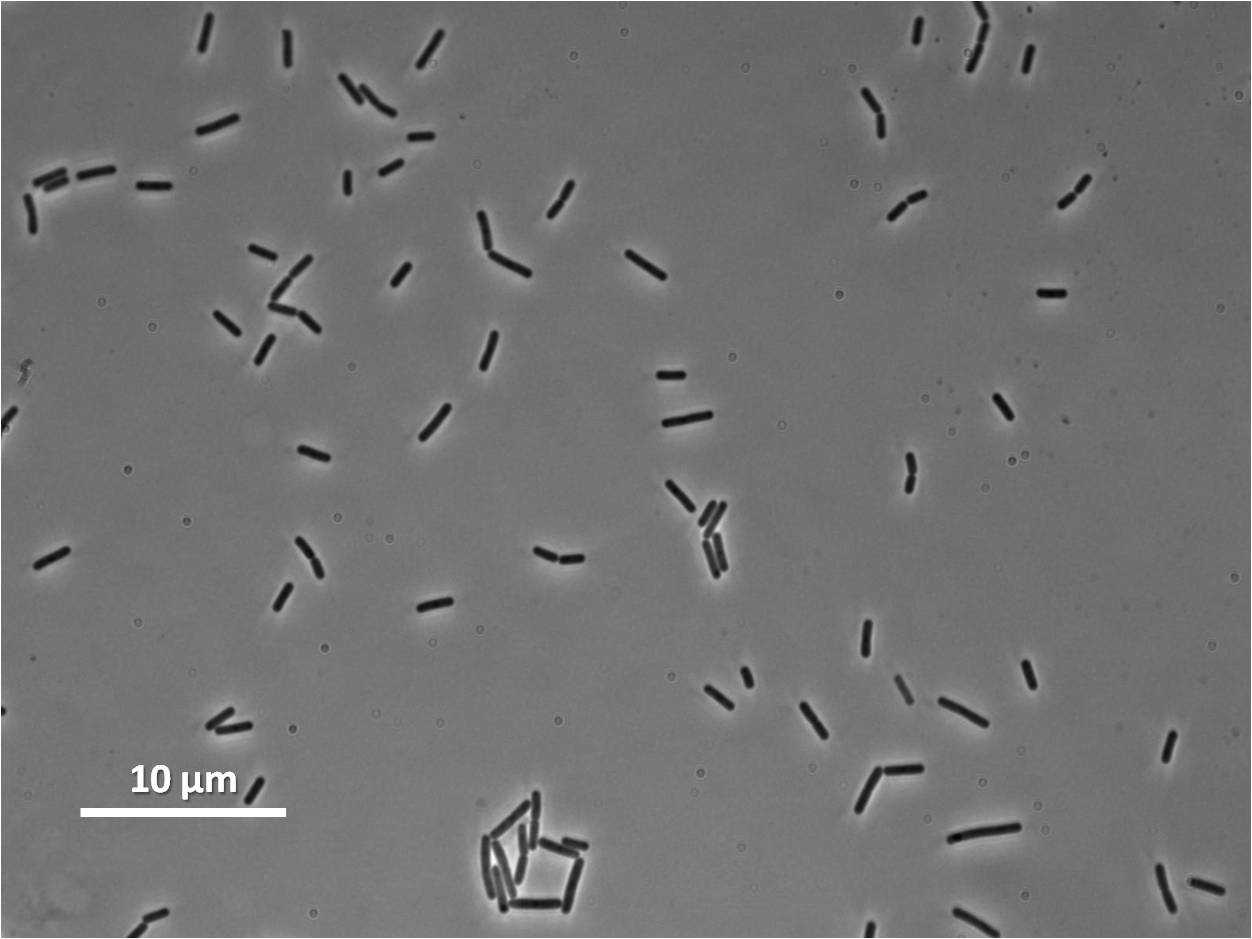
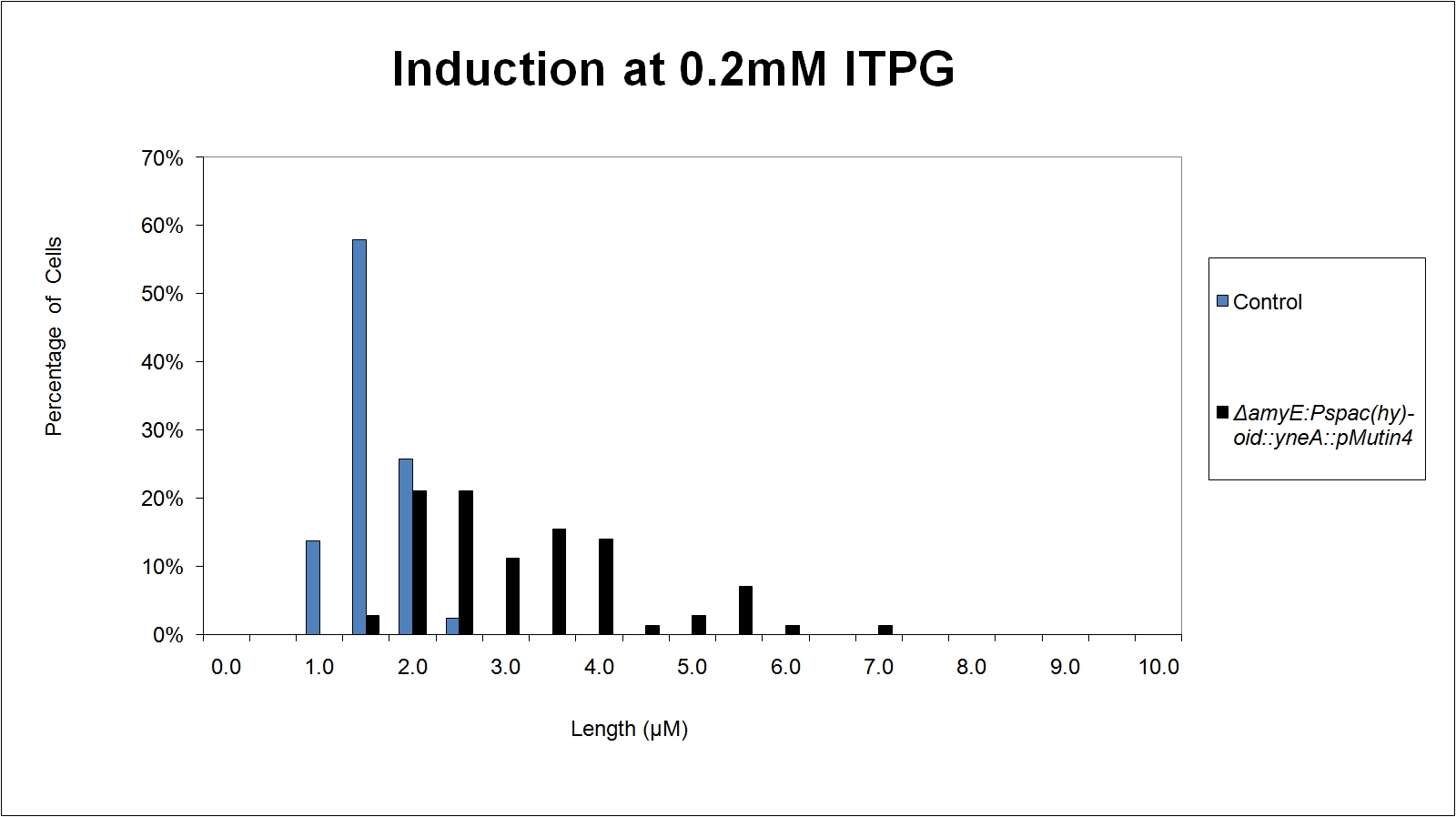
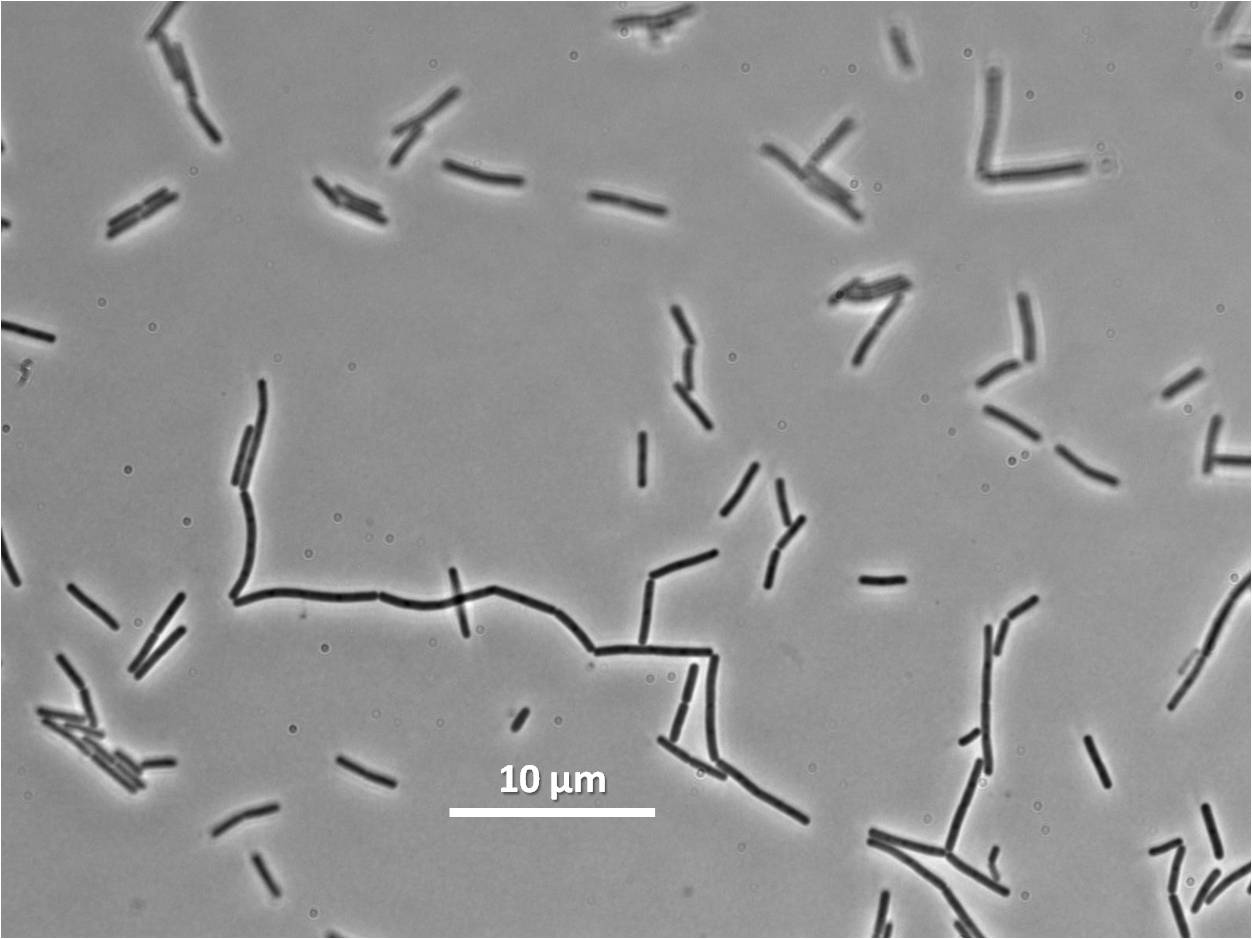
Graphs
Graphs 2,3 and 4 show a greater proportion of cells at a higher concentration of IPTG(1mM IPTG), compared with Bacillus subtilis 168 our control population.
References
Kawai, Y., Moriya, S., & Ogasawara, N. (2003). Identification of a protein, YneA, responsible for cell division suppression during the SOS response in Bacillus subtilis. Molecular microbiology, 47(4), 1113-22.
Mo, A.H. & Burkholder, W.F., (2010). YneA , an SOS-Induced Inhibitor of Cell Division in Bacillus subtilis , Is Regulated Posttranslationally and Requires the Transmembrane Region for Activity ᰔ †. Society, 192(12), 3159-3173. "
"