Team:Newcastle/23 August 2010
From 2010.igem.org
Shethharsh08 (Talk | contribs) (→pSB1C3 plasmid gel electrophoresis by adding EtBr) |
Shethharsh08 (Talk | contribs) |
||
| Line 60: | Line 60: | ||
| - | = | + | =Amplification of Pspac_oid promoter by PCR= |
| - | == | + | ==Aim== |
| - | The aim of this experiment is to | + | The aim of this experiment is to amplify Pspac_oid promoter fragment from plasmid pMK-RQ containing Biobrick ''kinA'' and plasmid pMK-RQ containing stochastic switch developed by Team Newcastle 2009 for the construction of [[Team:Newcastle/Urease|''rocF'' BioBrick]] with the help of 4 different Phusion PCR. |
==Materials and Protocol== | ==Materials and Protocol== | ||
| - | Please refer to | + | Please refer to [[Team:Newcastle/PCR| PCR]] for Phusion PCR protocol. The details for the 2 PCR reactions are mentioned below: |
| - | + | ===PCR=== | |
| - | = | + | {|border=1 |
| + | |- | ||
| + | !'''Tube''' | ||
| + | !'''Part to be amplified''' | ||
| + | !'''DNA fragment consisting the part''' | ||
| + | !'''Forward primer''' | ||
| + | !'''Reverse Primer''' | ||
| + | !'''Melting Temperature (Tm in °C) ''' | ||
| + | !'''Size of the fragment (in bp)''' | ||
| + | !'''Extension time* (in seconds)''' | ||
| + | |- | ||
| + | |1 | ||
| + | |Pspacoid PromoterPlease refer to [[Team:Newcastle/PCR| PCR]] for Phusion PCR protocol. The details for the 2 PCR reactions are mentioned below: | ||
| + | ===PCR=== | ||
| - | ''' | + | {|border=1 |
| - | + | |- | |
| - | + | !'''Tube''' | |
| - | + | !'''Part to be amplified''' | |
| - | + | !'''DNA fragment consisting the part''' | |
| - | + | !'''Forward primer''' | |
| - | * ''' | + | !'''Reverse Primer''' |
| - | + | !'''Melting Temperature (Tm in °C) ''' | |
| - | * ''' | + | !'''Size of the fragment (in bp)''' |
| + | !'''Extension time* (in seconds)''' | ||
| + | |- | ||
| + | |1 | ||
| + | |Pspacoid Promoter | ||
| + | |Plasmid containing ''kinA'' | ||
| + | |P1P1 forward | ||
| + | |P2P1 reverse | ||
| + | |58 | ||
| + | |106 approx. | ||
| + | |15 | ||
| + | |- | ||
| + | |2 | ||
| + | |Pspacoid Promoter | ||
| + | |Plasmid containing ''kinA'' | ||
| + | |P1P1 forward | ||
| + | |P2P1 reverse | ||
| + | |59 | ||
| + | |106 approx. | ||
| + | |15 | ||
| + | |- | ||
| + | |3 | ||
| + | |Pspacoid Promoter | ||
| + | |Plasmid containing stochastic switch | ||
| + | |P1P1 forward | ||
| + | |P2P1 reverse | ||
| + | |58 | ||
| + | |106 approx. | ||
| + | |15 | ||
| + | |- | ||
| + | |1 | ||
| + | |Pspacoid Promoter | ||
| + | |Plasmid containing stochastic switch | ||
| + | |P1P1 forward | ||
| + | |P2P1 reverse | ||
| + | |59 | ||
| + | |106 approx. | ||
| + | |15 | ||
| + | |} | ||
| + | |||
| + | '''Table 2''': Table represents 2 different Phusion PCR reactions for the amplification of Pspac_oid promoter, so that it can be ligated together with other fragments for the construction of ''rocF'' with the help of Gibson Cloning method. | ||
| + | * The extension rate of the Phusion polymerase is 1Kb/ 30 seconds. Thus the extension time of each and every PCR reaction is slightly different. | ||
| + | * For learning about the ''rocF'' fragments, please refer to the [[Media:Cloning_strategy_for_rocF.pdf|Cloning strategy for ''rocF'']]. | ||
==Discussion== | ==Discussion== | ||
| - | + | All the 4 Phusion PCR reactions were done however, gel electrophoresis will be done later today, to check whether the fragments have actually amplified or not. | |
==Conclusion== | ==Conclusion== | ||
| - | + | Today afternoon, we would be running gel electrophoresis to check the outcome of the 4 PCR reactions and later all the fragments will be ligated with help of Gibson protocol if the fragments have amplified. | |
| + | |||
{{Team:Newcastle/footer}} | {{Team:Newcastle/footer}} | ||
Revision as of 23:47, 24 August 2010

| |||||||||||||
| |||||||||||||
Contents |
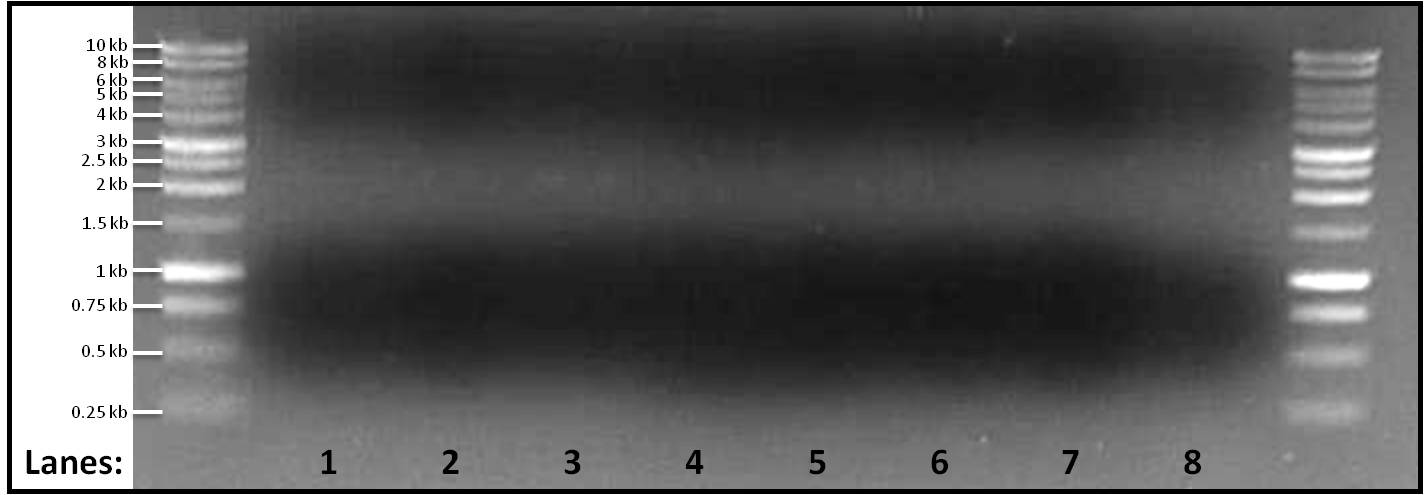
pSB1C3 plasmid gel electrophoresis
Aims
The aim of this experiment is to run gel electrophoresis for the extracted and linearized plasmid pSB1C3 fragment which were amplified at 4 different melting temperatures by 4 separate PCR reactions.
Materials and Protocol
Please refer to: gel electrophoresis for gel electrophoresis protocol.
Result
Figure 1: Gel electrophoresis of the amplified linearized plasmid pSB1C3 fragments ran at 4 different melting temperatures, Tms, (50, 60, 65, 70°C). A 1 kb DNA ladder was used on either side of lanes.
- Lane 1: pSB1C3 fragment amplified at 55°C
- Lane 2: pSB1C3 fragment amplified at 60°C
- Lane 3: pSB1C3 fragment amplified at 65°C
- Lane 4: pSB1C3 fragment amplified at 70°C
- Lane 5: pSB1C3 fragment amplified at 55°C
- Lane 6: pSB1C3 fragment amplified at 60°C
- Lane 7: pSB1C3 fragment amplified at 65°C
- Lane 8: pSB1C3 fragment amplified at 70°C
Discussion
No bands were found in any of the lanes. Yesterday, a faint band was found when the melting temperature was set at 65°C but today no band is found in lane 3 and lane 7. This makes finding the cause for no amplification even difficult. We would still be looking into it and would be changing other parameters.
Conclusion
The PCR reaction failed as there is no amplification found in any of the reactions.
pSB1C3 plasmid gel electrophoresis by adding EtBr
Aims
The aim of this experiment is to run gel electrophoresis for the extracted and linearized plasmid pSB1C3 fragment which were amplified at 4 different melting temperatures by 4 separate PCR reactions and to put Ethidium Bromoide (EtBr) in the agarose gel instead of safeview die so as to get better resolution and brighter bands.
Materials and Protocol
Please refer to: gel electrophoresis for gel electrophoresis protocol.
Please note that in this protocol we have made a minor modification which is that instead of adding 5µl of safeview dye, we have added 5µl of EtBr into the agarose gel so as to get better resolution and brighter bands.
Result
Figure 2: Gel electrophoresis (containing EtBr) of the amplified linearized plasmid pSB1C3 fragments ran at 4 different melting temperatures, Tms, (50, 60, 65, 70°C). A 1 kb DNA ladder was used on either side of lanes.
- Lane 1: pSB1C3 fragment amplified at 55°C
- Lane 2: pSB1C3 fragment amplified at 60°C
- Lane 3: pSB1C3 fragment amplified at 65°C
- Lane 4: pSB1C3 fragment amplified at 70°C
- Lane 5: pSB1C3 fragment amplified at 55°C
- Lane 6: pSB1C3 fragment amplified at 60°C
- Lane 7: pSB1C3 fragment amplified at 65°C
- Lane 8: pSB1C3 fragment amplified at 70°C
Discussion
No bands were found in any of the lanes. Yesterday, a faint band was found when the melting temperature was set at 65°C but today no band is found in lane 3 and lane 7. This makes finding the cause for no amplification even difficult. We would still be looking into it and would be changing other parameters.
Conclusion
The PCR reaction failed as there is no amplification found in any of the reactions.
Amplification of Pspac_oid promoter by PCR
Aim
The aim of this experiment is to amplify Pspac_oid promoter fragment from plasmid pMK-RQ containing Biobrick kinA and plasmid pMK-RQ containing stochastic switch developed by Team Newcastle 2009 for the construction of rocF BioBrick with the help of 4 different Phusion PCR.
Materials and Protocol
Please refer to PCR for Phusion PCR protocol. The details for the 2 PCR reactions are mentioned below:
PCR
| Tube | Part to be amplified | DNA fragment consisting the part | Forward primer | Reverse Primer | Melting Temperature (Tm in °C) | Size of the fragment (in bp) | Extension time* (in seconds) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pspacoid PromoterPlease refer to PCR for Phusion PCR protocol. The details for the 2 PCR reactions are mentioned below:
PCR
Table 2: Table represents 2 different Phusion PCR reactions for the amplification of Pspac_oid promoter, so that it can be ligated together with other fragments for the construction of rocF with the help of Gibson Cloning method.
DiscussionAll the 4 Phusion PCR reactions were done however, gel electrophoresis will be done later today, to check whether the fragments have actually amplified or not. ConclusionToday afternoon, we would be running gel electrophoresis to check the outcome of the 4 PCR reactions and later all the fragments will be ligated with help of Gibson protocol if the fragments have amplified.
|
 "
"