Team:NYMU-Taipei/Project/Speedy protein degrader
From 2010.igem.org
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Contents |
Abstract
- We developed the fluorescent proteins with degradation tags which accelerate the reporter turnover. This provides better temporal resolution of gene expression profiling in single cells.
A central goal of synthetic biology is to explore the design principles that embedded in the architecture and operation of biological systems. To understand how biological systems are genetically assembled, synthetic biologists need tools to quantitatively describe the gene expression in model organisms. Fluorescent proteins, along with modern microscopes, allows real-time gene expression profiling at the level of individual living cells. Though the detection of fluorophores benefits from their stability, this advantage turns awkward when we are monitoring the dynamic cellular events which required measurements at higher temporal resolution. One approach to achieve such demand is specific and accelerated reporter degradation. Thus, we focus on the design of fusion proteins with degradation tags. Previous studies suggest the LVA tag has hitherto been most efficient tag to shorten the half-life of tagged protein in Escherichia coli. We added LVA tags to the C-terminal of different fluorescent proteins. Our results indicate that the half-life of fluorophores are significantly reduced. Therefore, we improved the temporal resolution of reporter proteins. This provides a promising tool for studying transient gene expression in the future.
Introduction
What is our goal?
Fluorescent proteins, for example, green fluorescent protein (GFP), are now widely used to visualize a protein’s distribution and dynamics in a subcellular compartment. The remarkable feature of the fluorescent proteins (FP) is that the fluorophore forms spontaneously (Chalfie et al., 1994) without the need of any substrate or cofactor (Heim et al., 1994). In addition, FP variants can be fused to virtually any protein of interest; thus, they can be used in many species for detection purposes within single living cells. FPs, together with modern microscopes, has become essential tools for studying spatial and temporal dynamics of cellular processes at high resolution.
The biophysical characteristics of fluorescent probes provide opportunities and, set limitations for fusion-protein studies. The fluorophore of GFP and its variants is contained within a barrel of beta-sheet protein (Ormo et al., 1996). This compact structure renders FPs with high photostability under a variety of conditions, even under treatment with protease (Chalfie et al., 1994). Once stable FPs formed, they are cleared from the cells in tens of hours (Andersen et al., 1998). This property allows prolonged imaging of cells, and easier detection when FPs accumulated. However, the stability of FPs limits its application in some studies which needs fast reporter turnover, for example, studies of transient gene expression. This also impedes the measurements of temporal expression pattern and behavior of proteins, because proteins at different stages of their lifetime are being detected. To overcome this limitation, we tried to develop destablized FPs.
There are several ways to generate FP variants with different spectral and expression properties. One approach is mutagenesis studies. Though this approach is fruitful (Delagrave et al., 1995; Heim et al., 1995; Zacharias et al., 2002), it seems impractical to screen possible mutations of each FP in ever-increasing FP libraries. Alternatively, we planed to control proteolysis by adding degradation tags to FPs (Andersen et al., 1998). This approach can be easily generalized to several FPs; thus, it provides extensibility to future applications.
Controlled protein degradation in bacteria
The flow of genetic information can—and sometimes need to — depend not only on its controlled synthesis but also equally on its controlled degradation. Indeed, protein degradation in natural systems is essential for cellular functions. Previous study showed that 2.7% of proteins are degraded per generation in Escherichia coli (E. coli.) growing in logarithmic phase (Fox and Brown, 1961). Control of protein turnover is required for cell cycle progression, signal transduction, and rapid responses to environmental challenges (Grunenfelder et al., 2001; Hengge-Aronis, 2002; Neher et al., 2003). Therefore, regulated degradation is expected to be critical for the development of synthetic circuits.
To control the half-life of specific proteins, we utilized the regulated proteolysis machineries naturally employed to bacterial systems. Regulated degradation is ubiquitous for prokaryotic and eukaryotic cells (Hershko and Ciechanover, 1998; Jenal and Hengge-Aronis, 2003), because they need to clear damaged or aberrant proteins while ignoring functional ones. A common strategy for substrate selection is adding degradation tags to target proteins. Then, certain proteases execute energy dependent degradation of those proteins. One prominent type of degradation tag in prokaryotes is the ssrA tag, a tmRNA encoded by ssrA gene. This tagging system is engaged in protein quality control throughout all eubacteria (Karzai et al., 2000). Though it was first described as a mechanism to clear obstructed ribosomes (Keiler et al., 1996), recent studies suggested ssrA-mediated tagging also plays a regulatory role in the expression of lac operon (Abo et al., 2000). This motivated us to control the protein expression via manipulating controlled proteolysis.
ssrA tag
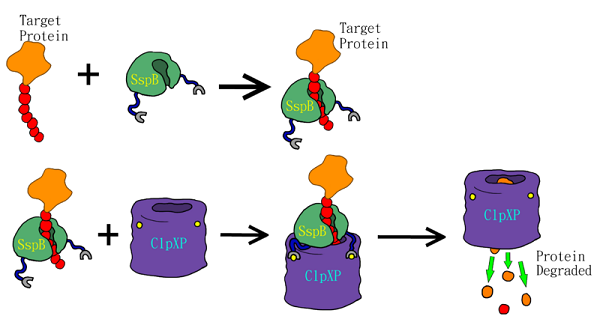
SsrA tag, encoded by the ssrA RNA, is important for protein quality control in bacteria. SsrA RNA is recognized as tmRNA because it has characteristics of both tRNA and mRNA (Atkins and Gesteland, 1996; Keiler et al., 1996). When E. coli. ribosomes stall during translation due to absence of a proper stop codon, ssrA ribosome-rescue system mediates C-terminal modification by adding the sequence AANDENYALAA to the the incomplete peptide (Keiler et al., 1996). SsrA tagging frees these ribosomes for other mRNAs, and targets the defective polypeptides for degradation by ClpXP and other ATP-dependent proteases (Fig.1)(Gottesman et al., 1998). Previous studies suggest that 0.5% of protein products in E. coli. receive ssrA tags (Lies and Maurizi, 2008). Bacterial strains lacking functional ssrA gene show slower growth (Oh and Apirion, 1991), reduction in motility (Komine et al., 1994), and subsided pathogenesis (Julio et al., 2000). These results indicate that ssrA tagging system play a major role in bacterial physiology.
The SsrA tag contains binding sites for the SspB adaptor and ClpXP protease (Levchenko et al., 2000). The SspB adaptor enhances substrate delivery by tethering SsrA-tagged proteins to the ClpX, which unfolds the tagged protein, and then translocates the denatured polypeptide into ClpP for degradation (Sauer et al., 2004). The binding affinity of the ssrA tag to the SspB and ClpX plays key role in modulating substrate choice (Flynn et al., 2004) and the efficiency of degradation (Hersch et al., 2004; McGinness et al., 2006). One of the mutants, called LVA tag, further accelerates the degradation of tagged proteins compared to the wild-type LAA tag (Andersen et al., 1998). LVA tag has been applied to construct a fast synthetic gene oscillator, while it decreases protein lifetime and increases temporal resolution (Stricker et al., 2008). Thus, we added the LVA tag to FPs to maximize the temporal resolution of these reporters.
Measurement
Data analysis
Experiment overview
What are we doing?
 "
"