Team:Mexico-UNAM-CINVESTAV/Project/Antifreeze Protein
From 2010.igem.org
(→Image:Antifreeze-protein.jpg) |
m (→Image:Antifreeze-protein.jpg) |
||
| (6 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Mexico-UNAM-CINVESTAV-HEADER}} | {{Mexico-UNAM-CINVESTAV-HEADER}} | ||
| - | |||
=[[Image:Antifreeze-protein.jpg]]= | =[[Image:Antifreeze-protein.jpg]]= | ||
| - | [[Image:Cristal.jpg| | + | [[Image:Cristal.jpg|300px||left]] |
| + | |||
| + | Antifreeze proteins (AFP's) is a class of polypeptides, with a polyphyletic ancestry, produced by certain animals (like insects and fish), plants and fungi that allows them to survive in freezing environments and to help them in maintaining the body fluidity. | ||
| + | |||
| + | Sea water in cold region freezes at -1.9C due to dissolved solutes. In order to prevent freezing at this temperatures, the animals blood must have a lower freezing point. The quality of AFP's to decrease the freezing point is not caused by changing the number of solute molecules. Instead, AFP’s bind to small ice crystals surfaces to inhibit recrystallization of ice in the cell membranes that would be fatal in animals | ||
| - | |||
| - | |||
| - | |||
| Line 56: | Line 56: | ||
*Are the hyperactive AFPs found in insects | *Are the hyperactive AFPs found in insects | ||
| - | *Throughout the length of the amino acid, at least every sixth is a cysteine residue. They are also hyperactive | + | *Throughout the length of the amino acid, at least every sixth is a cysteine residue. They are also hyperactive. |
| Line 62: | Line 62: | ||
*They have much weaker thermal hysteresis activity when compared to other AFPs. | *They have much weaker thermal hysteresis activity when compared to other AFPs. | ||
| - | *Their physiological function is likely in inhibiting the recrystallization of ice rather than in the preventing ice formation | + | *Their physiological function is likely in inhibiting the recrystallization of ice rather than in the preventing ice formation. |
| Line 68: | Line 68: | ||
'''''Mechanisms of action''''' | '''''Mechanisms of action''''' | ||
| - | AFP's | + | AFP's possess the ability to bind to the ice crystals surface, inhibiting the formation of ice. The growing ice surface becomes energetically unfavorable for further absorption of water molecules as the surface curvature increases, leading to the stopping of ice growth. This AFP-induced inhibition of ice crystal growth can be detected macroscopically as a drop in the freezing temperature (Tf) of the solution without alteration of the melting temperature (Tm) ([Tf –Tm]). This is called thermal hysteresis. (Chattopadhyay; 2007) (Davies and Hew; 1990) |
| - | Various processes are involved in the formation of solid phase of the water, among others, the interaction between ions or molecules leads to the formation of | + | Various processes are involved in the formation of solid phase of the water, among others, the interaction between ions or molecules leads to the formation of nuclei. The nucleation is the formation of new centers from which spontaneous growth can occur; this processes determine the size and distribution of the crystals. Large crystals may eventually be formed from fine crystals by a process called ripening. There are a lot of ions and other particles inside the cells. So when the temperature decreases the nucleation process is triggered in the cytoplasm. |
| + | <center>The following video is a simulation of the AFP used in this project with water molecules. | ||
| + | </center> | ||
| + | <html> | ||
| + | <center> | ||
| + | <iframe title="YouTube video player" class="youtube-player" type="text/html" width="425" height="349" src="http://www.youtube.com/embed/Fr03hNPt354?rel=0" frameborder="0"></iframe> | ||
| + | </center> | ||
| + | </html> | ||
--------------------------------------------------------------------------------------------------------------------------------------- | --------------------------------------------------------------------------------------------------------------------------------------- | ||
Latest revision as of 03:27, 28 October 2010
Antifreeze proteins (AFP's) is a class of polypeptides, with a polyphyletic ancestry, produced by certain animals (like insects and fish), plants and fungi that allows them to survive in freezing environments and to help them in maintaining the body fluidity.
Sea water in cold region freezes at -1.9C due to dissolved solutes. In order to prevent freezing at this temperatures, the animals blood must have a lower freezing point. The quality of AFP's to decrease the freezing point is not caused by changing the number of solute molecules. Instead, AFP’s bind to small ice crystals surfaces to inhibit recrystallization of ice in the cell membranes that would be fatal in animals
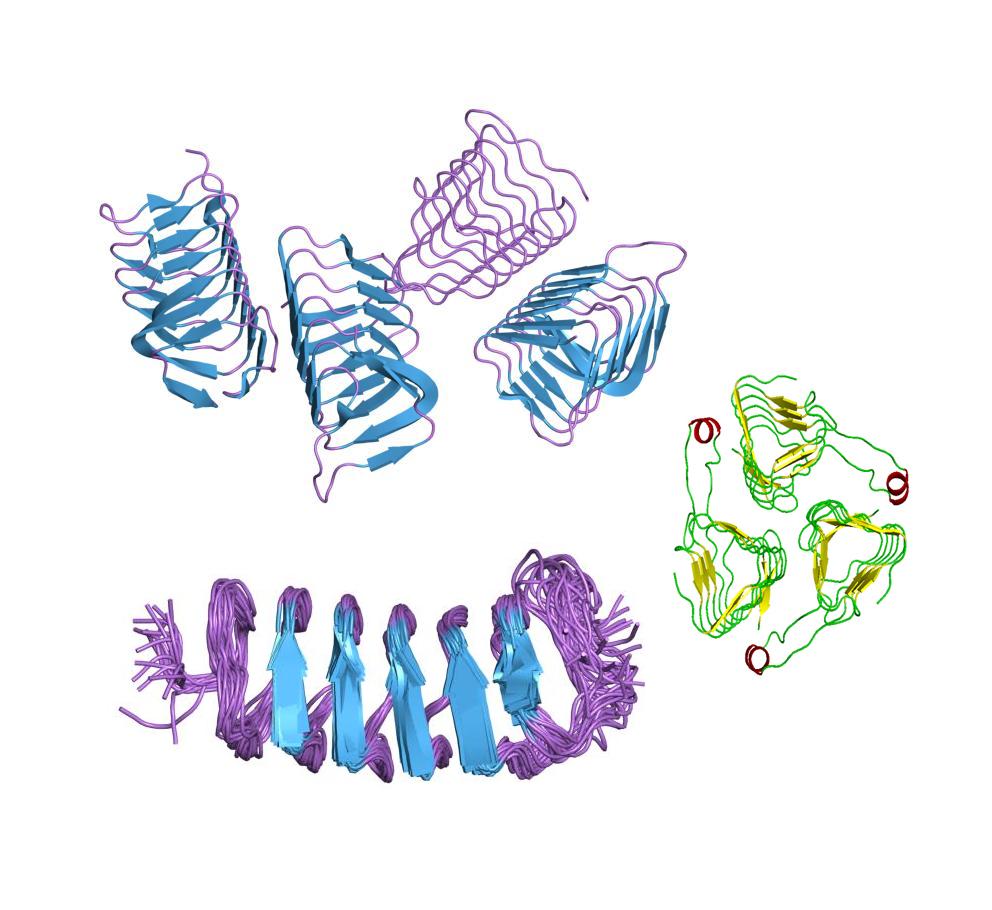
There are many kinds of AFP's synthesize by different organisms:
Type I AFP
- Found in winter flounder, longhorn sculpin and shorthorn sculpin.
- Consist of a single, long, amphipathic alpha helix 4kD approximately.
Type I-hyp AFP (where hyp stands for hyperactive)
- Found in righteye flounders.
- It is considerably better at depressing freezing temperature than most fish AFPs
Type II AFP
- Found in sea raven, smelt and herring.
- They are cysteine-rich globular proteins containing five disulfide bonds
Type III AFP
- Found in Antarctic eelpout.
- They exhibit similar overall hydrophobicity at ice binding surfaces to type I AFPs.
Type IV AFP
- Are found in longhorn sculpins.
- They are alpha helical proteins rich in glutamate and glutamine.
Type V AFP
- Are the hyperactive AFPs found in insects
- Throughout the length of the amino acid, at least every sixth is a cysteine residue. They are also hyperactive.
Plant AFP
- They have much weaker thermal hysteresis activity when compared to other AFPs.
- Their physiological function is likely in inhibiting the recrystallization of ice rather than in the preventing ice formation.
Mechanisms of action
AFP's possess the ability to bind to the ice crystals surface, inhibiting the formation of ice. The growing ice surface becomes energetically unfavorable for further absorption of water molecules as the surface curvature increases, leading to the stopping of ice growth. This AFP-induced inhibition of ice crystal growth can be detected macroscopically as a drop in the freezing temperature (Tf) of the solution without alteration of the melting temperature (Tm) ([Tf –Tm]). This is called thermal hysteresis. (Chattopadhyay; 2007) (Davies and Hew; 1990)
Various processes are involved in the formation of solid phase of the water, among others, the interaction between ions or molecules leads to the formation of nuclei. The nucleation is the formation of new centers from which spontaneous growth can occur; this processes determine the size and distribution of the crystals. Large crystals may eventually be formed from fine crystals by a process called ripening. There are a lot of ions and other particles inside the cells. So when the temperature decreases the nucleation process is triggered in the cytoplasm.
Chattopadhyay M. K; (2007); Antifreeze Proteins of Bacteria; Resonance p 25-30[1]
Davies, Peter L. and Hew, Choy L.; (1990); Biochemistry of fish antifreeze proteins; The FASEB journal; Vol. 4 p 2460-2468[2]
Scotter, A. J.; et al. (2006). "The basis for hyperactivity of antifreeze proteins". Cryobiology 53 (2): 229–239
 |
 |
 |
 |
 "
"