Team:Mexico-UNAM-CINVESTAV/Project
From 2010.igem.org
(→Image:Antifreeze-protein.jpg) |
(→Nucleation) |
||
| Line 44: | Line 44: | ||
| - | [[Image:Antifreeze-protein.jpg]] | + | =[[Image:Antifreeze-protein.jpg]]= |
Revision as of 15:55, 25 October 2010
One of the most important plants in the agriculture of Mexico is the coffee. The coffee cultivation in Mexico has to be in altitudes between the 900m to 1800m over the sea level, this is a problem when the frost of high mountains occurs because the coffee plant dies and thousands of acres lost. As the coffee is one of the most important plants in the economy of Mexico is a problem we must stop. (de Camargo; 2008)
At molecular level the cells have some issues with some basic functions. The transport of intracellular molecules to the outside of the cell; the rigidity of the membranes increase; decrease of the molecular, genetic and enzymatic kinetics; and the nucleation of ice crystals in the cytoplasm, membrane and extracellular medium. (D’amico, et al; 2006)
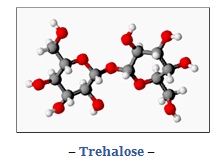
To avoid these problems, some organisms have developed different strategies in their cells. Certain organisms, as tardigrades and some fungus among others, use sugars, specially the disaccharide trehalose because it stabilizes the enzymes, proteins and membranes dehydrated. The trehalose interferes in the formation of ice crystals in the lipids and proteins bonds, binding as the water molecules to the lipid headgroup and the proteins about to denaturalize and keep them in their stabilized form. This make the membrane remains fluent and permeable so it stays alive. (Leslie, et al; 1995)
Other organisms developed diverse proteins that helps in the traduction and transcription of the genome. Binding to the RNA or DNA and help them to transcribe or tanslate the information. (D’amico, et al; 2006) This is the case of the Cold Shock Inducible Proteins (CSIP). These ones overexpressed when the cold shock begans and the bacteria is in the acclimation phase. It's like they are preparing the machinery to growth well at low temperature.
(Draw image by Daniel)
Another strategy that the eukaryotes use is to insert cholesterol molecules in the membrane, also the porkaryotes increased content of large lipid head groups. So the fluidity in both cases increase and the membrane does not become fragile and easy to break. (D’amico, et al; 2006) The last strategy, in which we are more interested, is the use of a group of singular proteins called Antifreeze Proteins, which are the model that we’re going to use in our project.
Before you keep going you must know about...
Nucleation
Various processes are involved in the formation of solid phase of the water, among others, the interaction between ions or molecules leads to the formation of nucleus. The nucleation is the formation of the new centers from which spontaneous growth can occur; this processes determines the size and distribution of produced crystals. Large crystals may eventually be formed from fine crystals by a process called ripening.There are a lot of ions and other particles Inside the cells. So when the temperature decreases the nucleation process arise in the cytoplasm.
Is a class of polypeptides, with a polyphyletic ancestry, produced by certain animals (like insects and fishes), plants and fungi that allow them to survive in freezing environments and to help them in maintain the body fluidity. (Simulation)
AFP`s possesses the ability to bind to the ice crystals surface, allowing the growth of ice at limited open spaces (ampliar) , leading to the formation of numerous convex ice surfaces between the bound of AFP`s. The growing ice surface becomes energetically unfavorable for further absorption of water molecules proportionately with the surface curvature, leading to the termination of ice growth. This AFP-induced inhibition of ice crystal growth can be detected macroscopically as a depression in the freezing temperature (Tf) of the solution without alteration of the melting temperature (Tm) ([Tf –Tm]). This is called thermal hysteresis. (Chattopadhyay; 2007) (Davies and Hew; 1990)
All organisms have different mechanisms in order to adapt to variable temperatures. Escherichia coli shows a mechanism denominated Cold Shock Response, which is important since at low temperatures the production of cytoplasmic proteins becomes blocked and E. coli cannot growth (Yamanaka; 1999).
The CSR consists in the expression of a specific set of proteins called cold shock proteins (Csp’s) to overcome this translational block. The cold shock protein A (cspA), which is the one we will use because is the one most studied protein of the family, has tree control processes involved with the system. The first control point is the auto regulation system Cold Box implicated with the cold shock protein. The second one is the 5’UTR mRNA region capability to become unstable at 37ºC. And the last one is at traductional level with the two ribosomes binding sites and an anti-DB sequence in the ribosome which allow to synthesize proteins at 15ºC or a little less (Yamanaka; 1999). But, how the expression of csp’s genes is able while the synthesis of most cytoplasm proteins is blocked? First, the cspA mRNA transcription is constitutive both at 37°C and 15°C, but the cold shock proteins expression is only present at low temperatures. This means that the mRNA is synthesized but the expression of proteins depends on the cspA mRNA sequence factors:
Promoter- cold box- 5’UTR- SD- met- DB
Promoter: The promoter consists of 50 bases. This promoter is active at both 37ºC and 15ºC but is more efficient at 15ºC than other promoters because it has an important UP element AT-rich that is report to be recognized by the subunit of RNA polymerase and which give it a high promoter activity.
Cold box: Is an important region of the 5’ UTR region, which may form a stable stem-loop structure and is implicated in the auto regulation of the cspA system.
5’ UTR: Is a region that consists of mostly 159 bases and is downstream the promoter. This region make the cspA mRNA unstable at 37º C with an average half-life of less than 12 seconds, but more stable at 15°C with an average half-life of 20 minutes and improve the translation at low temperature. In here there is an RNAse cleavage site immediately upstream the Shine-Dalgarno sequence that is considered to be responsible for this unstable messenger stage.
Shine-Dalgarno (SD) sequence: Is a four-nucleotide sequence that the ribosome uses to bind the mRNA and is common for many messengers also the ones that we use in the IGEM competition. (AAGG)
DB sequence: Is a fourteen nucleotide sequence that is in the mRNA and is complementary to a region found in the 16S rRNA named as anti-DB sequence , which gives the feature of good translation at low temperature because it function as an extra messenger ribosome binding site. In this way the ribosome can anchor the SD sequence and the DB sequence as a two ribosome-binding site to permit a great cleavage between the ribosome and the messenger. (Yamanaka; 1999) (Baneyx and Mujacic; 2002)
Also the cell has many other proteins that are involved in the protein, DNA or RNA stabilization at low temperatures: RecA, which is involved in recombination and induction of the cold shock response; H-NS, a nucleotide-associated DNA-binding protein, which is required for optimal growth at low temperature; GyrA, the subunit of the topoisomerase DNA gyrase, which helps to uncoil the double helix of the DNA; NusA, which is involved in termination of transcription; PNP, which is an exoribonuclease; and Hsc66, a Hsp70 homologue to cold (Thieringer, et al; 1998) (Yamanaka; 1999).
PROJECT
In our project, we’re trying to combine two of the strategies that nature has developed to overcome the problem of cells and tissues freezing. First we organize a cold regulation system (we’re going to explain it ahead) at transcriptional and translational levels using a constitutive gene that Escherichia coli uses to respond at 15 ºC up to 10ºC. Also we had added an antifreeze protein, in order to help the cell to avoid that the water goes under freezing process arising strong hydrogen bounds and maintaining a vitrification process over a normal crystallizing of water process. In that way the cytoplasm and the cell membrane did not be damage at low temperatures. This both strategies may create new biobricks that can function as a “cold kit” to both synthesize proteins and build an Escherichia coli mutant that allow us to work with it at low temperatures. Even we are trying to overcome other human issues like the plants and crops maintenance, in order to survive the cold weather countries like Canada, USA, Northern European countries and of course the frosts occurring in the north of Mexico and high mountains of country. In order to improve efficiency to our cold system we build four constructions to prove the different kind of regulation that the mechanism can allow.
• Firstly we took the promoter with all the parts involved, from the promoter polymerase-binding zone, to the Downstream Box that is inside the protein DNA sequence.
• In a second construction we took all the regulation system except the promoter to define if another promoter different to the CspA can be regulated with the freeze system.

• We also have the CspA promoter alone so we can put it controlling another gene and see if this can function at low temperatures.
• For a final construction, we took the Cold Box, the UTR region and the Shine-Dalgarno sequence with the exception of the Downstream Box and the promoter since we are not sure if having two methionines at the beginning of the protein shall cause problems to the translational system in the ribosomes, even if it’s an important point of regulation in the freeze system. Despite we have founded in some reports that the freeze system could be express under these conditions, we are trying to confirm this and to valuate which construction is better.

To make these constructions we will use primers that allow us to make PCR and obtain the different pieces of the Cold Shock promoter. These primers have been designed with the specifics requirements, so they have segments of cutting restriction enzymes that the Biobrick Standard Assembly 10 request. This allow us to cut and paste anywhere we want as a biobrick; using this we also can do the constructions with the AFP that we want, and express the protein at low temperatures. To characterize the promoter we use the Relative Promoter Units (RPU). This consist in proving the biobricks with GFP and see their expresion compared to an stablished promotor that is already tested. With this we can asure that our promoter works fine or is not quite good, and we can. Our biobrick have been located in the pSB3K3 kanamycin vector with the E0240 GFP. The promoter I20260 have been located in the same vector with the same GFP than us (E0240) and then we compare both ones.
To send the biobricks we use the pSB1C3 chloramphenicol vector as the Biobrick Standard Assembly 10 request.
The diverse constructions of the promoter in biobrick format with the AFP have been settled in the pSb1A2 ampycilin vector to express and prove that the biobricks are working as we want. This is our final construction and is the one we are expecting to serve as an antifreeze system.
Machinery that regulates the expression + AFP = "E.coldi"
"http://upload.wikimedia.org/wikipedia/commons/0/08/Walschaerts_motion.gif"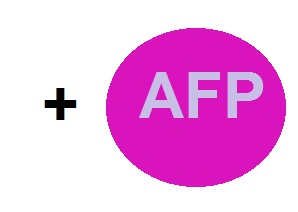

Baneyx, François and Mujacic, Mirna; (2002); Cold-Inducible Promoters for Heterologous Protein Expression; from Methods in Molecular Biology; Vol. 205 Humana Press Inc.[http://www.springerlink.com/content/qx66q8875x0q7043/#section=86530&page=1]
D’amico, Salvino, et al; (2006); Psychrophilic microorganisms: challenges for life; European Molecular Biology Organization Vol.7 No. 4 p 385-389[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1456908/]
de Camargo, A. P. and de Camargo, M. B. P. (2008) Frost in Coffee Crops: Frost Characteristics, Damaging Effects on Coffee and Alleviation Options, in Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers (ed J. N. Wintgens), Wiley-VCH Verlag GmbH, Weinheim, Germany. doi: 10.1002/9783527619627.ch11
Chattopadhyay M. K; (2007); Antifreeze Proteins of Bacteria; Resonance p 25-30[http://www.ias.ac.in/resonance/December2007/p25-30.pdf]
Davies, Peter L. and Hew, Choy L.; (1990); Biochemistry of fish antifreeze proteins; The FASEB journal; Vol. 4 p 2460-2468[http://www.fasebj.org/cgi/content/abstract/4/8/2460]
Heather A. Thieringer, Pamela G. Jones, and Masayori Inouye; (1998); Cold Shock and Adaptation; Bio Essays 20.1: 40-57[http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1521-1878(199801)20:1%3C49::AID-BIES8%3E3.0.CO;2-N/abstract]
Leslie Samuel B., et al; (1995);Trehalose and Sucrose Protect Both Membranes and Proteins in Intact Bacteria during Drying; Applied and Environmental Microbiology; Vol. 61, No. 10[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC167656/pdf/613592.pdf]
Pankow J. F. (1991) Aquatic Chemistry Concept, EUA CRC Press LLC. p.683[http://books.google.com.mx/books?hl=es&lr=&id=bV3BZVSw-lYC&oi=fnd&pg=PA3&dq=Aquatic+Chemistry+Concept&ots=o0GQl0B_Ng&sig=ncS8JTnfM3GEG1_hO-6SV2YqqwE]
Yamanaka, Kunitoshi; (1999); Cold Shock Response in Escherichia coli; Journal of Molecular and Microbiological Biotechnology 1(2): 193-202[http://www.horizonpress.com/jmmb/v1/v1n2/03.pdf]
 |
 |
 |
 |
 "
"