Team:MIT mammalian Mechanosensation
From 2010.igem.org
(Difference between revisions)
| (14 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
<ul> | <ul> | ||
<li><a href="https://2010.igem.org/Team:MIT_toggle">Overview</a></li> | <li><a href="https://2010.igem.org/Team:MIT_toggle">Overview</a></li> | ||
| + | <li><a href="https://2010.igem.org/Team:MIT_tmodel">Modelling</a></li> | ||
<li><a href="https://2010.igem.org/Team:MIT_tconst">Toggle Construction</a></li> | <li><a href="https://2010.igem.org/Team:MIT_tconst">Toggle Construction</a></li> | ||
| - | <li><a href=" | + | <li><a href="https://2010.igem.org/Team:MIT_composite">Characterization</a></li> |
</ul> | </ul> | ||
</dd> | </dd> | ||
| Line 60: | Line 61: | ||
<div id="unique" style="padding:0px; font-size: 14px; border: 1px solid black; margin:0px; background-color:transparent;"> | <div id="unique" style="padding:0px; font-size: 14px; border: 1px solid black; margin:0px; background-color:transparent;"> | ||
| - | <table width=650px style="background-color: white; margin-top:5px; padding: 10px;"><tr><td><div class="bodybaby">The Search for Mechanosensitive Promoters</div></td> | + | <table width=650px style="background-color: white; margin-top:5px; padding: 10px;"><tr><td> |
| + | <div class="outline"> | ||
| + | <a href="#lit">1 Literature Search</a><br> | ||
| + | <a href="#pcloning">2 Promoter Construction</a><br> | ||
| + | <a href="#Experiments">3 Experimental Outline</a><br> | ||
| + | <ul><a href="#Shaker"> Shaker Experiment </a><br> | ||
| + | <ul><a href="#sdescrip">Experimental Set Up</a><br> | ||
| + | <a href="#sresults">Results</a><br> | ||
| + | <a href="#sconclusion">Conclusion</a><br> | ||
| + | </ul> | ||
| + | <a href="#Micro"> Microfluidic Experiments </a><br> | ||
| + | <ul><a href="#mdescrip">Experimental Set Up</a><br> | ||
| + | <a href="#mseed">Device Seeding</a><br> | ||
| + | <a href="#mSS">Applying Fluid Shear Stress</a><br> | ||
| + | <a href="#mstretch"> Applying Nonuniform Cyclic Strain</a><br> | ||
| + | <a href="#mconclusion"> Conclusion</a><br> | ||
| + | </ul> | ||
| + | </ul> | ||
| + | <a href="#full">4 Our Full Toggle</a><br> | ||
| + | <ul><a href="#model"> Model </a><br></ul> | ||
| + | </div></td> | ||
| + | |||
| + | <tr><td><div class="bodybaby">The Search for Mechanosensitive Promoters</div></td> | ||
<tr><td> | <tr><td> | ||
| - | <b class="bolded" id=" | + | <b class="bolded" id="lit">Literature Search </b><br> |
<br> | <br> | ||
To begin, we searched the literature for well-characterized mechanosensitive promoters; we restricted our pool of candidates to promoters that had been shown, mostly by upregulation of a fluorescent marker, to be directly responsive to mechanical stress. The nature of the promoters varied. We included several short oligonucleotide sequences SRE/CRE2 and NR1/2, that had been shown to confer sensitivitiy to shear stress when placed in front of a ‘dummy’ SV40 promoter. Promoters for the genes PAI-2 and cfos were identified as mechanosensitive in the literature; we used portions of these promoters which were shown to be responsible for the mechanosensitivity. Click <a href = "https://static.igem.org/mediawiki/2010/e/e1/FocalAdhesionsNotes.pdf">here</a> and <a href = "https://static.igem.org/mediawiki/2010/3/3d/ShearStressNotes.pdf">here</a> for more in-depth notes on our literature review. | To begin, we searched the literature for well-characterized mechanosensitive promoters; we restricted our pool of candidates to promoters that had been shown, mostly by upregulation of a fluorescent marker, to be directly responsive to mechanical stress. The nature of the promoters varied. We included several short oligonucleotide sequences SRE/CRE2 and NR1/2, that had been shown to confer sensitivitiy to shear stress when placed in front of a ‘dummy’ SV40 promoter. Promoters for the genes PAI-2 and cfos were identified as mechanosensitive in the literature; we used portions of these promoters which were shown to be responsible for the mechanosensitivity. Click <a href = "https://static.igem.org/mediawiki/2010/e/e1/FocalAdhesionsNotes.pdf">here</a> and <a href = "https://static.igem.org/mediawiki/2010/3/3d/ShearStressNotes.pdf">here</a> for more in-depth notes on our literature review. | ||
| - | <div class="bodybaby" id="pcloning">Promoter | + | <div class="bodybaby" id="pcloning">Promoter Construction</div><br> |
<br> | <br> | ||
We used PCR to amplify the relevant regions of the PAI-2 and cfos promoters from human genomic DNA; the short oligonucleotide sequences SRE/CRE2 and NR1/2 were added in front of an SV40 ‘dummy’ promoter, with appropriate primers. We then used three-way Gateway recombination (see our <a href = "https://2010.igem.org/Team:MIT_mammalian_Standard">new standardization for mammalian cloning</a>) to construct a lentiviral plasmid that contained the promoter of interest controlling EGFP expression. We used these constructs to transiently transfect cells with in the presence of helper viral plasmids; this yielded a virus containing our construct of interest. We then infected HEK cells with these constructs and selected with the appropriate antibiotic for two weeks to create stable cell lines. Several of the experiments done below were carried out before creation of the stable cell lines; to quickly obtain cell lines containing the relevant construct, we transiently transfected cells with the construct and no viral helper plasmids. Using that protocol, the construct did not get integrated into the genome, but it allowed us to carry out short term characterization assays. | We used PCR to amplify the relevant regions of the PAI-2 and cfos promoters from human genomic DNA; the short oligonucleotide sequences SRE/CRE2 and NR1/2 were added in front of an SV40 ‘dummy’ promoter, with appropriate primers. We then used three-way Gateway recombination (see our <a href = "https://2010.igem.org/Team:MIT_mammalian_Standard">new standardization for mammalian cloning</a>) to construct a lentiviral plasmid that contained the promoter of interest controlling EGFP expression. We used these constructs to transiently transfect cells with in the presence of helper viral plasmids; this yielded a virus containing our construct of interest. We then infected HEK cells with these constructs and selected with the appropriate antibiotic for two weeks to create stable cell lines. Several of the experiments done below were carried out before creation of the stable cell lines; to quickly obtain cell lines containing the relevant construct, we transiently transfected cells with the construct and no viral helper plasmids. Using that protocol, the construct did not get integrated into the genome, but it allowed us to carry out short term characterization assays. | ||
| Line 78: | Line 101: | ||
<br><br> | <br><br> | ||
<div class="bodybaby" id="Shaker">Shaker Experiments</div><br><br> | <div class="bodybaby" id="Shaker">Shaker Experiments</div><br><br> | ||
| + | |||
<b class="bolded" id="sdescrip">Experimental Setup </b><br><br> | <b class="bolded" id="sdescrip">Experimental Setup </b><br><br> | ||
| + | <img src=https://static.igem.org/mediawiki/2010/3/3c/0_shaker_outline.png> | ||
Transient tranfections of pMech_EGFP constructs were performed on HEK293FT cells seeded at 2 million cells/well in two identically seeded six-well plates. The constructs used were: pCOFS_EGFP, pPAI-2_EGFP, pSRE/CRE2_SV40_EGFP, pNR1/2_SV40_EGFP, and CMV_EGFP. Of these CMV_EGFP served as a control for transformation efficiency. 12 hours after transfection one of the two six well plates was designated as "shear stress" and placed on a shaker at 120 rpm inside the incubator. The other plate was designated "control" and not subjected to shaking. Fluorescent micrographs were obtained on a Zeiss microscope for each well at 100, 215, and 500 ms exposure times every 3 hours. Brightfield images were exposed for 6 ms. Images were exported from the accompanying Axiovision software at identical histogram levels for each cell line. | Transient tranfections of pMech_EGFP constructs were performed on HEK293FT cells seeded at 2 million cells/well in two identically seeded six-well plates. The constructs used were: pCOFS_EGFP, pPAI-2_EGFP, pSRE/CRE2_SV40_EGFP, pNR1/2_SV40_EGFP, and CMV_EGFP. Of these CMV_EGFP served as a control for transformation efficiency. 12 hours after transfection one of the two six well plates was designated as "shear stress" and placed on a shaker at 120 rpm inside the incubator. The other plate was designated "control" and not subjected to shaking. Fluorescent micrographs were obtained on a Zeiss microscope for each well at 100, 215, and 500 ms exposure times every 3 hours. Brightfield images were exposed for 6 ms. Images were exported from the accompanying Axiovision software at identical histogram levels for each cell line. | ||
| Line 84: | Line 109: | ||
<br><br> | <br><br> | ||
| + | <HR> | ||
<b class="bolded" id="sresults">Results </b><br><br> | <b class="bolded" id="sresults">Results </b><br><br> | ||
| - | <a href="https://static.igem.org/mediawiki/2010/5/5c/CFOS_shaker.png" class="thickbox" title="CFOS Promoter Experimental Results"><img src="https://static.igem.org/mediawiki/2010/5/5c/CFOS_shaker.png" width=300px></a> | + | <a href="https://static.igem.org/mediawiki/2010/5/5c/CFOS_shaker.png" class="thickbox" title="CFOS Promoter Experimental Results. CFOS was not expected to show response to shear."><img src="https://static.igem.org/mediawiki/2010/5/5c/CFOS_shaker.png" width=300px></a> |
| - | <a href="https://static.igem.org/mediawiki/2010/b/b7/SRE_shaker.png" class="thickbox" title="SRE Promoter Experimental Results"><img src="https://static.igem.org/mediawiki/2010/b/b7/SRE_shaker.png" width=300px></a> | + | <a href="https://static.igem.org/mediawiki/2010/b/b7/SRE_shaker.png" class="thickbox" title="SRE Promoter Experimental Results. SRE showed >2x increase in activity in response to shear."><img src="https://static.igem.org/mediawiki/2010/b/b7/SRE_shaker.png" width=300px></a> |
| - | <a href="https://static.igem.org/mediawiki/2010/0/04/PAI2_shaker.png" class="thickbox" title="SRE Promoter Experimental Results"><img src="https://static.igem.org/mediawiki/2010/0/04/PAI2_shaker.png" width=300px></a> | + | <a href="https://static.igem.org/mediawiki/2010/0/04/PAI2_shaker.png" class="thickbox" title="SRE Promoter Experimental Results. PAI2 was not expected to show response to shear."><img src="https://static.igem.org/mediawiki/2010/0/04/PAI2_shaker.png" width=300px></a> |
| - | <a href="https://static.igem.org/mediawiki/2010/3/3d/2_shaker.png" class="thickbox" title="SRE Promoter Experimental Results"><img src="https://static.igem.org/mediawiki/2010/3/3d/2_shaker.png" width=300px></a> | + | <a href="https://static.igem.org/mediawiki/2010/3/3d/2_shaker.png" class="thickbox" title="SRE Promoter Experimental Results. SRE showed 2x increase in activity in response to shear."><img src="https://static.igem.org/mediawiki/2010/3/3d/2_shaker.png" width=300px></a> |
| + | <p> | ||
| + | <img src=https://static.igem.org/mediawiki/2010/6/6b/Promoters-bar-graph.png> | ||
| + | <p>Bar graph showing the ratio of fluorescence to cell, normalized to the 2.5hr fluorescence to cell ratio. | ||
<br><br> | <br><br> | ||
| - | <b class="bolded" id=" | + | <HR> |
| + | <b class="bolded" id="sconclusion">Conclusion </b><br><br> | ||
Our data show that SRE/CRE_SV40 and NR1/2_SV40 promoters were significantly upregulated in response to shear stress as compared to negative controls (R_o's of around 2 or greater). PAI-2 showed high R_o at T=8, but overall very low absolute EGFP expression. CFOS showed similar expression levels for both shear stress and negative control. | Our data show that SRE/CRE_SV40 and NR1/2_SV40 promoters were significantly upregulated in response to shear stress as compared to negative controls (R_o's of around 2 or greater). PAI-2 showed high R_o at T=8, but overall very low absolute EGFP expression. CFOS showed similar expression levels for both shear stress and negative control. | ||
| Line 109: | Line 139: | ||
<a href="https://static.igem.org/mediawiki/2010/6/6a/Flow_channel_microfluidic.jpg" class="thickbox" title="2-Layer Microfluidic Device Diagram"><img src="https://static.igem.org/mediawiki/2010/6/6a/Flow_channel_microfluidic.jpg" width=300px></a> | <a href="https://static.igem.org/mediawiki/2010/6/6a/Flow_channel_microfluidic.jpg" class="thickbox" title="2-Layer Microfluidic Device Diagram"><img src="https://static.igem.org/mediawiki/2010/6/6a/Flow_channel_microfluidic.jpg" width=300px></a> | ||
| + | <HR> | ||
<b class="bolded" id="mseed">Device Seeding </b><br><br> | <b class="bolded" id="mseed">Device Seeding </b><br><br> | ||
| - | Our first move was to test cell seeding inside the microfluidic devices we had manufactured; below (top) are micrographs imaged on day 4 of healthy HEK cells growing in shear stress single channel devices. Below (bottom) is a video of HEK cells being seeded into the devices. | + | Our first move was to test cell seeding inside the microfluidic devices we had manufactured; below (top left and top right) are micrographs imaged on day 4 of healthy CHO and HEK cells, respectively, growing in shear stress single channel devices. Below (bottom) is a video of HEK cells being seeded into the devices. |
<br><br> | <br><br> | ||
| - | + | <table> | |
| - | <a href="https://static.igem.org/mediawiki/2010/d/d6/CHO_microfluidics.jpg | + | |
| - | <a href="https://static.igem.org/mediawiki/2010/7/7a/HEK_microfluidics.jpg | + | <tr> |
| + | <td>CHO</td><td>HEK</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | <td><a href="https://static.igem.org/mediawiki/2010/d/d6/CHO_microfluidics.jpg"><img src="https://static.igem.org/mediawiki/2010/c/cc/Cho_ufl.jpg" width=300px></a></td><td><a href="https://static.igem.org/mediawiki/2010/7/7a/HEK_microfluidics.jpg"><img src="https://static.igem.org/mediawiki/2010/1/13/Hek_ufl.jpg" width=300px></a></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
<br><br> | <br><br> | ||
<object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/Mjp6vA814WE?fs=1&hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/Mjp6vA814WE?fs=1&hl=en_US&rel=0" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | <object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/Mjp6vA814WE?fs=1&hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/Mjp6vA814WE?fs=1&hl=en_US&rel=0" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | ||
| - | <b class="bolded" id=" | + | <HR> |
| + | <b class="bolded" id="mSS">Microfluidic Experiments with Mechanosensitive Promoters: Fluid Shear Stress</b><br><br> | ||
We ran several trial experiments seeding the shear stress devices with reporter cell lines containing EGFP under the control of one of our mechanosensitive promoters. We were lucky enough to work in a lab that had a confocal microscope available 24/7; we applied shear stress to the device for two hours, then imaged every 15 minutes to obtain a timecourse of promoter activity in response to fluid shear stress. | We ran several trial experiments seeding the shear stress devices with reporter cell lines containing EGFP under the control of one of our mechanosensitive promoters. We were lucky enough to work in a lab that had a confocal microscope available 24/7; we applied shear stress to the device for two hours, then imaged every 15 minutes to obtain a timecourse of promoter activity in response to fluid shear stress. | ||
| Line 127: | Line 168: | ||
<a href="https://static.igem.org/mediawiki/2010/c/cd/Photo_63.jpg" class="thickbox" title="Microfluidic Device Logistical Setup"><img src="https://static.igem.org/mediawiki/2010/c/cd/Photo_63.jpg" width=300px></a> | <a href="https://static.igem.org/mediawiki/2010/c/cd/Photo_63.jpg" class="thickbox" title="Microfluidic Device Logistical Setup"><img src="https://static.igem.org/mediawiki/2010/c/cd/Photo_63.jpg" width=300px></a> | ||
<br><br> | <br><br> | ||
| - | + | Our first attempts at applying shear stress washed away a portion of the cell layer; the remaining cells began to expand and divide over the last half of the timecourse. Below is a video of the channel over a 12 hour period - cells were stimulated with fluid shear stress for 1 hour and 40 minutes, then imaged every 15 minutes to obtain a timecourse of fluorescence. | |
<object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/c5NEadvJY-k?fs=1&hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/c5NEadvJY-k?fs=1&hl=en_US&rel=0" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | <object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/c5NEadvJY-k?fs=1&hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/c5NEadvJY-k?fs=1&hl=en_US&rel=0" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | ||
| - | <b class="bolded" id=" | + | <HR> |
| - | We then constructed a series of 'cell stretching' devices and checked to see if oscillating media through the flow channels could significantly strain the cell monolayer. | + | <b class="bolded" id="mstretch">Deformable Substrate Pilot Assay: Nonuniform Cyclic Strain</b><br><br> |
| + | We then tested our second assay, to measure the response to cell membrane stretching. We then constructed a series of 'cell stretching' devices and checked to see if oscillating media through the flow channels could significantly strain the cell monolayer. | ||
Below is a video taken of the a cyclically stretched HEK cell monolayer, taken on Day 4 after seeding into the microfluidic device. . | Below is a video taken of the a cyclically stretched HEK cell monolayer, taken on Day 4 after seeding into the microfluidic device. . | ||
<object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/-qcy6z44aFo?fs=1&hl=en_US"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/-qcy6z44aFo?fs=1&hl=en_US" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | <object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/-qcy6z44aFo?fs=1&hl=en_US"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/-qcy6z44aFo?fs=1&hl=en_US" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | ||
| - | + | <p> | |
| + | <HR> | ||
| + | <b class="bolded" id="mconclusion">Conclusion</b><br><br> | ||
| + | <br><br> | ||
| + | We have created stable cell lines containing four different mechanosensitive promoters, and assayed promoter function in a shaker experiment. We've also built and tested a microfluidic apparatus for applying fluid shear stress and stretching forces to the cells. We've also cloned constructs containing all of the mechanosensitive promoters controlling the expression of BMP2 protein; we now have the parts to create and test stable cell lines with potentially mechanosensitive BMP2 secretion. | ||
</td></table> | </td></table> | ||
Latest revision as of 03:55, 28 October 2010
The Search for Mechanosensitive Promoters |
||||
|
Literature Search To begin, we searched the literature for well-characterized mechanosensitive promoters; we restricted our pool of candidates to promoters that had been shown, mostly by upregulation of a fluorescent marker, to be directly responsive to mechanical stress. The nature of the promoters varied. We included several short oligonucleotide sequences SRE/CRE2 and NR1/2, that had been shown to confer sensitivitiy to shear stress when placed in front of a ‘dummy’ SV40 promoter. Promoters for the genes PAI-2 and cfos were identified as mechanosensitive in the literature; we used portions of these promoters which were shown to be responsible for the mechanosensitivity. Click here and here for more in-depth notes on our literature review. Promoter Construction We used PCR to amplify the relevant regions of the PAI-2 and cfos promoters from human genomic DNA; the short oligonucleotide sequences SRE/CRE2 and NR1/2 were added in front of an SV40 ‘dummy’ promoter, with appropriate primers. We then used three-way Gateway recombination (see our new standardization for mammalian cloning) to construct a lentiviral plasmid that contained the promoter of interest controlling EGFP expression. We used these constructs to transiently transfect cells with in the presence of helper viral plasmids; this yielded a virus containing our construct of interest. We then infected HEK cells with these constructs and selected with the appropriate antibiotic for two weeks to create stable cell lines. Several of the experiments done below were carried out before creation of the stable cell lines; to quickly obtain cell lines containing the relevant construct, we transiently transfected cells with the construct and no viral helper plasmids. Using that protocol, the construct did not get integrated into the genome, but it allowed us to carry out short term characterization assays. Experimental Outlines We planed to tested the sensitivity of our candidate promoters to an array of mechanical stresses. To accomplish this, we created and tested cell lines containing fluorescent reporter constructs for each promoter, and observed changes in fluorescence after the cells were exposed to mechanical stimulation. We applied shear stress with two different methods; the first involved periodic vibration of the cell plate on automated shaker. For the second method, we worked with a students in Roger Kamm's lab to build microfluidic devices; we seeded cells inside the devices and flowed media through the channels to approximate fluid shear stress. Shaker Experiments Experimental Setup  Transient tranfections of pMech_EGFP constructs were performed on HEK293FT cells seeded at 2 million cells/well in two identically seeded six-well plates. The constructs used were: pCOFS_EGFP, pPAI-2_EGFP, pSRE/CRE2_SV40_EGFP, pNR1/2_SV40_EGFP, and CMV_EGFP. Of these CMV_EGFP served as a control for transformation efficiency. 12 hours after transfection one of the two six well plates was designated as "shear stress" and placed on a shaker at 120 rpm inside the incubator. The other plate was designated "control" and not subjected to shaking. Fluorescent micrographs were obtained on a Zeiss microscope for each well at 100, 215, and 500 ms exposure times every 3 hours. Brightfield images were exposed for 6 ms. Images were exported from the accompanying Axiovision software at identical histogram levels for each cell line.
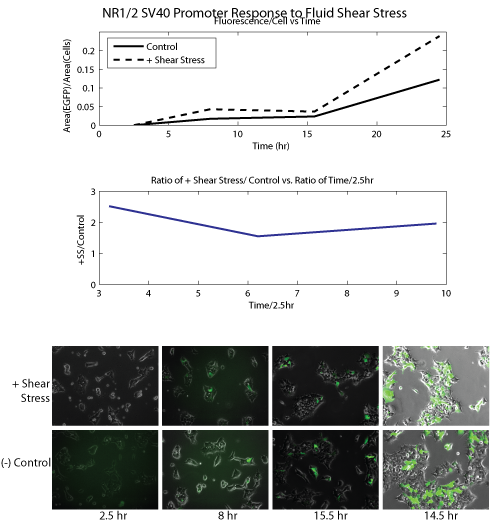
For image analysis, accurate cell count could not be obtained because HEK cells grow at high density. Instead area occupied by cells was used as an analogous measurement for fields of view with similar % confluence and measured with imageJ, an image analysis software provided by the NIH. Fluorescent micrographs were processed using the binary function in imageJ, followed by particle analysis to obtain the total area occupied by fluorescence. The ratio of the area of fluorescence and the area of cells were calculated (R_fl) for time points 2.5h, 8h, 15.5h, and 24.5h. The overall ratio R_o was calculated as R_fl(T)/R_fl(2.5), where T=8,15.5, or 24.5. In this process the image levels and cell confluence of each micrograph were carefully matched for comparison. Graphic representation of our data can be seen in the figures below.
Transient tranfections of pMech_EGFP constructs were performed on HEK293FT cells seeded at 2 million cells/well in two identically seeded six-well plates. The constructs used were: pCOFS_EGFP, pPAI-2_EGFP, pSRE/CRE2_SV40_EGFP, pNR1/2_SV40_EGFP, and CMV_EGFP. Of these CMV_EGFP served as a control for transformation efficiency. 12 hours after transfection one of the two six well plates was designated as "shear stress" and placed on a shaker at 120 rpm inside the incubator. The other plate was designated "control" and not subjected to shaking. Fluorescent micrographs were obtained on a Zeiss microscope for each well at 100, 215, and 500 ms exposure times every 3 hours. Brightfield images were exposed for 6 ms. Images were exported from the accompanying Axiovision software at identical histogram levels for each cell line.
For image analysis, accurate cell count could not be obtained because HEK cells grow at high density. Instead area occupied by cells was used as an analogous measurement for fields of view with similar % confluence and measured with imageJ, an image analysis software provided by the NIH. Fluorescent micrographs were processed using the binary function in imageJ, followed by particle analysis to obtain the total area occupied by fluorescence. The ratio of the area of fluorescence and the area of cells were calculated (R_fl) for time points 2.5h, 8h, 15.5h, and 24.5h. The overall ratio R_o was calculated as R_fl(T)/R_fl(2.5), where T=8,15.5, or 24.5. In this process the image levels and cell confluence of each micrograph were carefully matched for comparison. Graphic representation of our data can be seen in the figures below.
Results 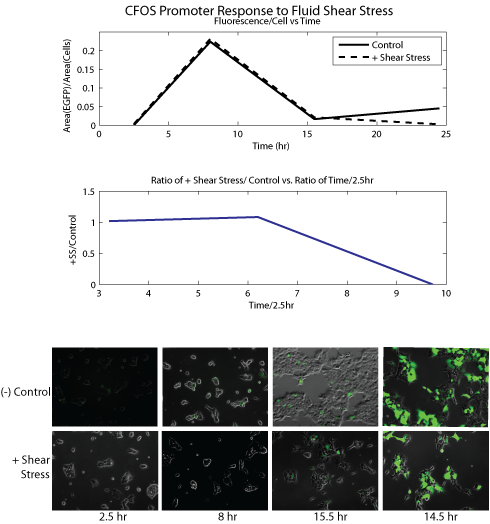


Bar graph showing the ratio of fluorescence to cell, normalized to the 2.5hr fluorescence to cell ratio.
Conclusion Our data show that SRE/CRE_SV40 and NR1/2_SV40 promoters were significantly upregulated in response to shear stress as compared to negative controls (R_o's of around 2 or greater). PAI-2 showed high R_o at T=8, but overall very low absolute EGFP expression. CFOS showed similar expression levels for both shear stress and negative control. The data strongly correlated with our initial literature research: SRE/CRE_SV40 and NR1/2_SV40 were promoters specifically shown to be responsive to shear stress, while PAI-2 and CFOS were mechanosensitive but not necessarily to stress, rather to strain or stretching. We concluded that our current sample of promoters was promising and proceeded to microfluidic experiments to further analyze the properties of these promoters. Microfluidic Experiments Experimental Setup We will use two different types of microfluidic devices, to assay the cellular response to fluid shear stress and cell layer ‘stretching’, respectively. However the two are not mutually exclusive; stretching of the cell layer could create interstitial flow, cross-activating the fluid shear stress response. Below on the left is a diagram of our shear stress devices. We flowed media over cells seeded in the channels of these devices to apply fluid shear stress. The devices used in our second assay, for cell membrane stretching, are shown on the right. We 'stretched' the cells by flowing media through channels beneath the cell monolayer; this deflected the monolayer upward and stretched the cell membrane. 

Device Seeding Our first move was to test cell seeding inside the microfluidic devices we had manufactured; below (top left and top right) are micrographs imaged on day 4 of healthy CHO and HEK cells, respectively, growing in shear stress single channel devices. Below (bottom) is a video of HEK cells being seeded into the devices.
Microfluidic Experiments with Mechanosensitive Promoters: Fluid Shear Stress We ran several trial experiments seeding the shear stress devices with reporter cell lines containing EGFP under the control of one of our mechanosensitive promoters. We were lucky enough to work in a lab that had a confocal microscope available 24/7; we applied shear stress to the device for two hours, then imaged every 15 minutes to obtain a timecourse of promoter activity in response to fluid shear stress. A picture of our experimental setup: 
Our first attempts at applying shear stress washed away a portion of the cell layer; the remaining cells began to expand and divide over the last half of the timecourse. Below is a video of the channel over a 12 hour period - cells were stimulated with fluid shear stress for 1 hour and 40 minutes, then imaged every 15 minutes to obtain a timecourse of fluorescence. Deformable Substrate Pilot Assay: Nonuniform Cyclic Strain We then tested our second assay, to measure the response to cell membrane stretching. We then constructed a series of 'cell stretching' devices and checked to see if oscillating media through the flow channels could significantly strain the cell monolayer. Below is a video taken of the a cyclically stretched HEK cell monolayer, taken on Day 4 after seeding into the microfluidic device. .
Conclusion We have created stable cell lines containing four different mechanosensitive promoters, and assayed promoter function in a shaker experiment. We've also built and tested a microfluidic apparatus for applying fluid shear stress and stretching forces to the cells. We've also cloned constructs containing all of the mechanosensitive promoters controlling the expression of BMP2 protein; we now have the parts to create and test stable cell lines with potentially mechanosensitive BMP2 secretion. |
 "
"
 iGEM 2010
iGEM 2010

