Team:Lethbridge/Notebook/Lab Work/May
From 2010.igem.org
Adam.smith4 (Talk | contribs) |
Liszabruder (Talk | contribs) |
||
| (47 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | <div style="background-color:#000000; color:white"> | |
| + | <html> | ||
| - | + | <br> | |
| - | + | <table border="0" width="100%" style="background-color:#000000"> | |
| - | + | <tr> | |
| - | + | <th> | |
| - | + | <image src="https://static.igem.org/mediawiki/2010/2/29/UofLteamlogo.jpg" width="200px"/> | |
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | <th> | |
| - | + | ||
| - | + | ||
| - | + | <image src="https://static.igem.org/mediawiki/2010/9/91/UofLLabWork.JPG" height="300px"/> | |
| - | + | ||
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | <th> | |
| - | + | ||
| - | + | ||
| - | + | <image src="https://static.igem.org/mediawiki/2010/2/29/UofLteamlogo.jpg" width="200px"/> | |
| - | + | ||
| - | + | ||
| - | + | </th> | |
| - | + | </tr> | |
| + | </table> | ||
| - | |||
| - | |||
| - | == | + | <br> |
| - | ===May 5/2010=== | + | |
| + | <align="centre"> | ||
| + | <table border="0" width="100%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <th> | ||
| + | |||
| + | <a href="https://2010.igem.org/Team:Lethbridge"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/2/22/UofLHome.jpg" width="80"/> | ||
| + | </a> | ||
| + | |||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Team"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/0d/UofLTeam.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Project"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/8d/UofLProjectbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Parts"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/84/UofLPartsSubmittedToTheRegistrybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Modeling"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/e/e1/UofLModelingbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Ethics"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/2/26/UofLEthicsbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Safety"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/00/UofLSafetybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Art"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/0a/UofLArt.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/News"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/c/c3/UofLNewsButton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | </table> | ||
| + | </body> | ||
| + | </html> | ||
| + | <hr> | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <font color="white">Feel free to look around our notebook! | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <body> | ||
| + | <center> | ||
| + | <table border="0" width="28%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | <th> | ||
| + | <div class="miniBar"> | ||
| + | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
| + | <div class="miniContainer"> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/9/91/UofLprotocolsbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Calendar"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLcalendar.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th> | ||
| + | <div class="miniBar"> | ||
| + | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=11&date_day=05&date_year=0&un=THE IGEM JAMBOREE&size=normal&mo=11&da=05&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=11&date_day=05&date_year=0&un=THE IGEM JAMBOREE&size=normal&mo=11&da=05&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
| + | <div class="miniContainer"> | ||
| + | </th> | ||
| + | <tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | <hr> | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <font color="white">Here you can check out the work we have done in the lab, click on a month to take a look! | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <body> | ||
| + | <center> | ||
| + | <table border="0" width="50%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/April"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/8a/UofLapril.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/May"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/7b/UofLmaybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/June"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/80/UofLjunebutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/July"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/5/53/UofLjulybutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/August"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/1/15/UofLaugustbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/September"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/4/4d/UofLseptemberbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/October"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/4/4e/UofLoctoberbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | |||
| + | |||
| + | <tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </body> | ||
| + | </html> | ||
| + | <hr> | ||
| + | |||
| + | <BLOCKQUOTE> | ||
| + | |||
| + | =<font color="white">May= | ||
| + | ==<font color="white">May 5/2010== | ||
(in the lab: JV)<br> | (in the lab: JV)<br> | ||
<b>Objective:</b> Test Restriction Endonucleases for activity (take 2)<br> | <b>Objective:</b> Test Restriction Endonucleases for activity (take 2)<br> | ||
| Line 56: | Line 211: | ||
Reactions will be assembled as follows:<br> | Reactions will be assembled as follows:<br> | ||
<table><table border="3"> | <table><table border="3"> | ||
| - | <tr><td><b>Enzyme<td>Buffer<td>Volume MM(µL)<td>Volume Enzyme(µL)</b> | + | <tr><td><b>Enzyme</b></td><td><b>Buffer</b></td><td><b>Volume MM(µL)</b></td><td><b>Volume Enzyme(µL)</b></td></tr> |
| - | <tr><td>PstI</td><td>Red</td><td>19.75</td><td>.25</td></tr> | + | <tr><td>PstI</td><td>Red</td><td>19.75</td><td>0.25</td></tr> |
| - | <tr><td>XbaI</td><td>Tango</td><td>19.75</td><td>.25</td></tr> | + | <tr><td>XbaI</td><td>Tango</td><td>19.75</td><td>0.25</td></tr> |
| - | <tr><td>SpeI</td><td>Tango</td><td>19.75</td><td>.25</td></tr> | + | <tr><td>SpeI</td><td>Tango</td><td>19.75</td><td>0.25</td></tr> |
| - | <tr><td>EcoRI</td><td>Red</td><td>19.75</td><td>.25</td></tr> | + | <tr><td>EcoRI</td><td>Red</td><td>19.75</td><td>0.25</td></tr> |
| - | <tr><td>EcoRI/SpeI</td><td>Red</td><td>19.5</td><td>.25+.25</td></tr> | + | <tr><td>EcoRI/SpeI</td><td>Red</td><td>19.5</td><td>0.25 + 0.25</td></tr> |
| - | <tr><td>XbaI/SpeI</td><td>Tango</td><td>19.5</td><td>.25+.25</td></tr> | + | <tr><td>XbaI/SpeI</td><td>Tango</td><td>19.5</td><td>0.25 + 0.25</td></tr> |
| - | <tr><td>EcoRI/PstI</td><td>Red</td><td>19.5</td><td>.25+.25</td></tr> | + | <tr><td>EcoRI/PstI</td><td>Red</td><td>19.5</td><td>0.25 + 0.25</td></tr> |
| - | <tr><td>XbaI/PstI</td><td>Tango</td><td>19.5</td><td>.25+.25</td></tr> | + | <tr><td>XbaI/PstI</td><td>Tango</td><td>19.5</td><td>0.25 + 0.25</td></tr> |
</table><br> | </table><br> | ||
Make up Master Mixes as follows:<br> | Make up Master Mixes as follows:<br> | ||
| Line 106: | Line 261: | ||
<b>Conclusion:</b> Test other source of SpeI to see if it has any activity.<br><br> | <b>Conclusion:</b> Test other source of SpeI to see if it has any activity.<br><br> | ||
| - | ===May 6/2010 | + | ==<font color="white">May 6/2010== |
| - | (in the lab:KG, AS)<br> | + | (in the lab: KG, AS)<br> |
<b>Objective:</b> To check if the old SpeI enzyme (exp date: March 2011) will cleave plasmid DNA, since we believe the newer SpeI enzyme (exp date: 2012) does not.<br> | <b>Objective:</b> To check if the old SpeI enzyme (exp date: March 2011) will cleave plasmid DNA, since we believe the newer SpeI enzyme (exp date: 2012) does not.<br> | ||
<b>Method:</b><br> | <b>Method:</b><br> | ||
| Line 141: | Line 296: | ||
S(N); SpeI(N)<br> | S(N); SpeI(N)<br> | ||
S(O); SpeI(O)<br><br> | S(O); SpeI(O)<br><br> | ||
| - | Placed in -20<sup>o</sup>C freezer of later analysis by agarose electrophoresis<br> | + | Placed in -20<sup>o</sup>C freezer of later analysis by agarose electrophoresis<br> |
| - | + | ||
| - | ===May 10/2010 | + | ==<font color="white">May 10/2010== |
(in the lab:JV)<br> | (in the lab:JV)<br> | ||
<b>Objective:</b> To analyze the restriction test done by KG and AS on May 6/2010 by agarose electrophoresis<br><br> | <b>Objective:</b> To analyze the restriction test done by KG and AS on May 6/2010 by agarose electrophoresis<br><br> | ||
| Line 176: | Line 330: | ||
Add 500µL of stock ampicillin to 500mL of media<br><br> | Add 500µL of stock ampicillin to 500mL of media<br><br> | ||
| - | ===May 11/2010 Evening | + | ==<font color="white">May 11/2010 Evening== |
| - | (in the lab: KG, AV, MC, TF, JV, JS)< | + | (in the lab: KG, AV, MC, TF, JV, JS)<br> |
<b>Objective:</b> To transform the following plasmids into DH5α <i>E.coli</i> cells. | <b>Objective:</b> To transform the following plasmids into DH5α <i>E.coli</i> cells. | ||
<table><table border="3"> | <table><table border="3"> | ||
| Line 216: | Line 370: | ||
<li>pTet</li></ul><br> | <li>pTet</li></ul><br> | ||
| - | ===May 12/2010 | + | ==<font color="white">May 12/2010== |
(in the lab: JV)<br> | (in the lab: JV)<br> | ||
<b>Objective: Miniprep of plasmid DNA from transformed cells</b>(JV, AV, HB)<br> | <b>Objective: Miniprep of plasmid DNA from transformed cells</b>(JV, AV, HB)<br> | ||
| Line 336: | Line 490: | ||
Start overnight cultures of cells that grew for plasmid prep and sequencing.<br><br> | Start overnight cultures of cells that grew for plasmid prep and sequencing.<br><br> | ||
| - | ===May 14/2010 | + | ==<font color="white">May 14/2010== |
(in the lab: JV)<br> | (in the lab: JV)<br> | ||
<b>Objective:</b> Quantify pDNA concentration in order to ensure sufficient material for sequence analysis.<br> | <b>Objective:</b> Quantify pDNA concentration in order to ensure sufficient material for sequence analysis.<br> | ||
| Line 408: | Line 562: | ||
There is plasmid DNA in each sample which, when cut with both the prefix and suffix enzyme, yields a band approximately 2000bp (size of pSB1A3 is 2157bp).<br><br> | There is plasmid DNA in each sample which, when cut with both the prefix and suffix enzyme, yields a band approximately 2000bp (size of pSB1A3 is 2157bp).<br><br> | ||
| - | ===May 17/2010 | + | ==<font color="white">May 17/2010== |
(in the lab: JV, AV)<br> | (in the lab: JV, AV)<br> | ||
Make agar plates with 100µg/mL of ampicillin<br> | Make agar plates with 100µg/mL of ampicillin<br> | ||
| Line 414: | Line 568: | ||
Make 13 x 5mL sterile liquid LB broth<br><br> | Make 13 x 5mL sterile liquid LB broth<br><br> | ||
| - | ===May 17/2010 Evening | + | ==<font color="white">May 17/2010 Evening== |
(in the lab: TF, AS)<br> | (in the lab: TF, AS)<br> | ||
<b>Objective:</b> To grow cells for future use<br> | <b>Objective:</b> To grow cells for future use<br> | ||
| Line 447: | Line 601: | ||
Only sRBS (D5) and pBad-TetR cells (from transformation plates) grew.<br> | Only sRBS (D5) and pBad-TetR cells (from transformation plates) grew.<br> | ||
| - | ===May 18/2010 | + | ==<font color="white">May 18/2010== |
(in the lab: JV, AV, HB)<br> | (in the lab: JV, AV, HB)<br> | ||
<b>NOTE:</b> Cells from liquid cultures grown last night (May 17/2010) were made into glycerol stocks and placed into the [[Team:Lethbridge/Notebook/Working_Glycerol_Stocks|working glycerol stock box]] as follows:<br> | <b>NOTE:</b> Cells from liquid cultures grown last night (May 17/2010) were made into glycerol stocks and placed into the [[Team:Lethbridge/Notebook/Working_Glycerol_Stocks|working glycerol stock box]] as follows:<br> | ||
| Line 524: | Line 678: | ||
Ran gel at 100V for 90 minutes.<br> | Ran gel at 100V for 90 minutes.<br> | ||
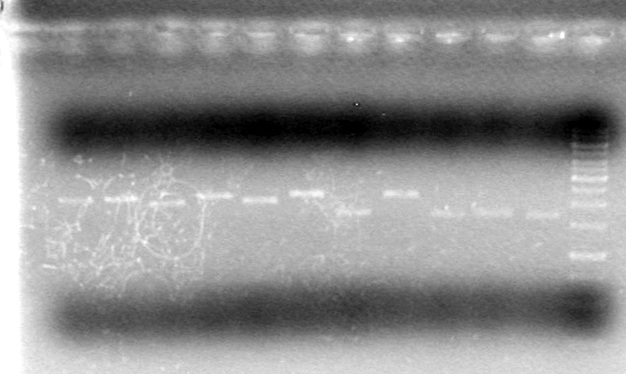
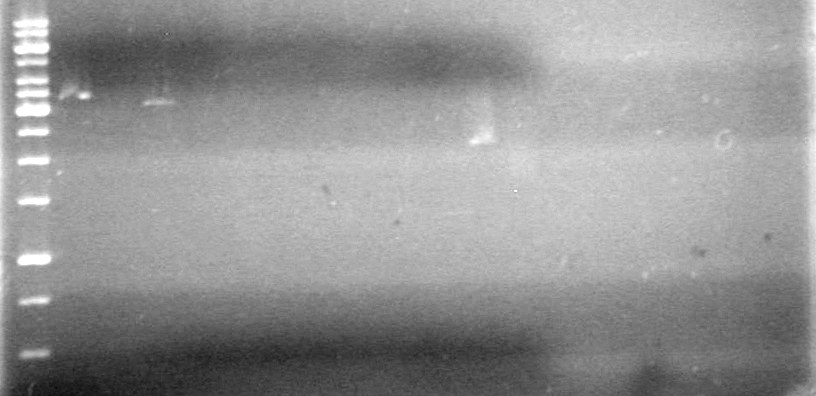
<b>Results:</b><br><br> | <b>Results:</b><br><br> | ||
| - | + | [[image:100518JV1.JPG|200px|none]] | |
<b>Objective:</b> Make liquid cultures of streak plates (made May 17/2010) for plasmid mini-preps.<br> | <b>Objective:</b> Make liquid cultures of streak plates (made May 17/2010) for plasmid mini-preps.<br> | ||
<b>Method:</b> | <b>Method:</b> | ||
| Line 540: | Line 694: | ||
<li>xylE (C4-2007 Box)</li></ul><br> | <li>xylE (C4-2007 Box)</li></ul><br> | ||
| - | ===May 18/2010 Evening | + | ==<font color="white">May 18/2010 Evening== |
(in the lab: KG)<br> | (in the lab: KG)<br> | ||
<b>Objective:</b> Restrict pLacI, sRNS, sRBS-Lumazine Synthase-dt out of plasmid then ligase pLacI and sRBS also pLacI sRBS-Lumazine Synthase-dt.<br> | <b>Objective:</b> Restrict pLacI, sRNS, sRBS-Lumazine Synthase-dt out of plasmid then ligase pLacI and sRBS also pLacI sRBS-Lumazine Synthase-dt.<br> | ||
| Line 603: | Line 757: | ||
<tr><tr>sRBS-Lumazine Synthase-dt</table><br> | <tr><tr>sRBS-Lumazine Synthase-dt</table><br> | ||
Begin room temperature incubation at 8:25pm. | Begin room temperature incubation at 8:25pm. | ||
| - | ===May 19 | + | |
| + | ==<font color="white">May 19/2010== | ||
(in the lab:JV)<br> | (in the lab:JV)<br> | ||
| Line 637: | Line 792: | ||
Reactions ran for 1 hour at 37<sup>o</sup>C.<br> | Reactions ran for 1 hour at 37<sup>o</sup>C.<br> | ||
| - | ===May 20 | + | ==<font color="white">May 20/2010== |
(in the lab:JV, AV)<br> | (in the lab:JV, AV)<br> | ||
| Line 671: | Line 826: | ||
</table><br> | </table><br> | ||
Ran gel at 100V for 2 hours<br> | Ran gel at 100V for 2 hours<br> | ||
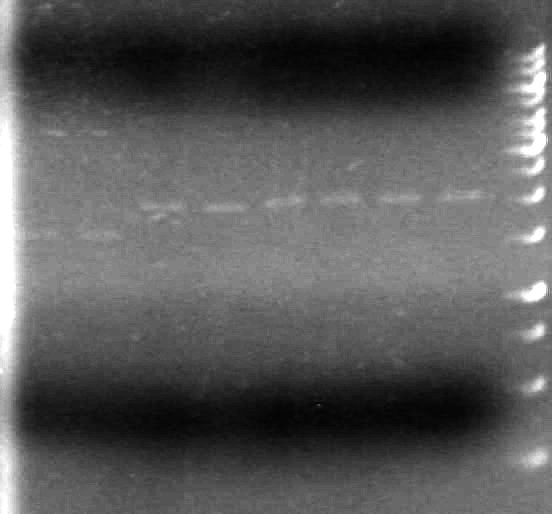
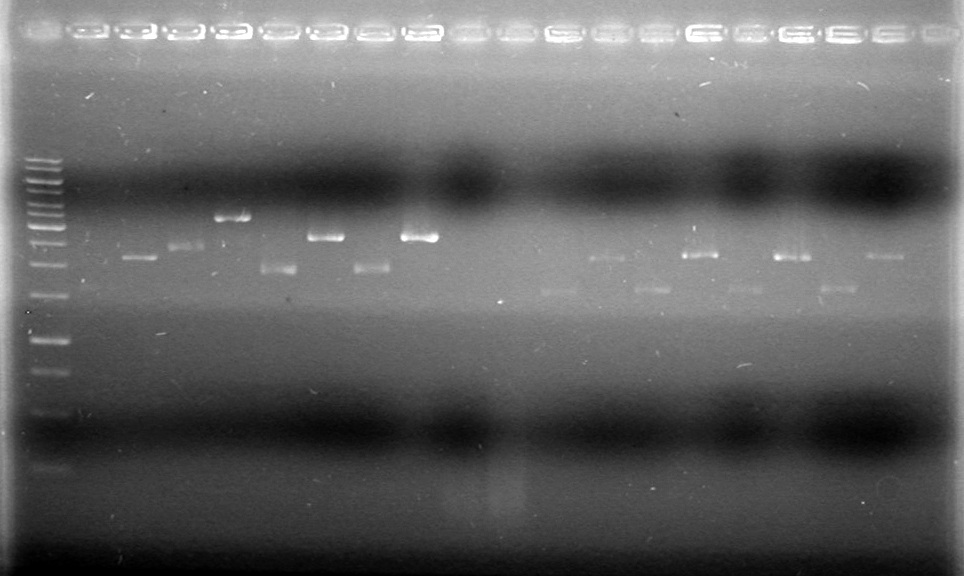
| - | ===May 20 | + | <b>Results:</b><br> |
| + | [[image:100520AV.JV-Plasmid Check.JPG|200px|none]] | ||
| + | |||
| + | ==<font color="white">May 20/2010 Evening== | ||
(in the lab:JS, AS)<br> | (in the lab:JS, AS)<br> | ||
| Line 707: | Line 865: | ||
Ran gel for 80 minutes at 100V.<br> | Ran gel for 80 minutes at 100V.<br> | ||
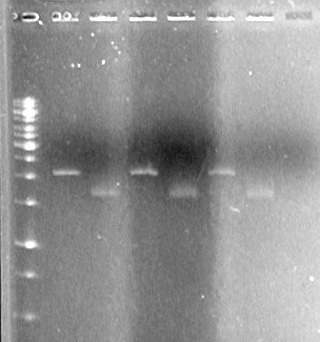
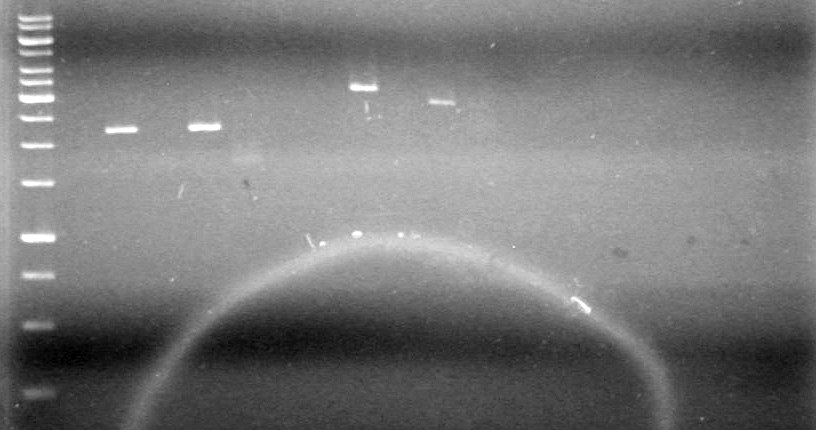
<b>Results:</b> | <b>Results:</b> | ||
| - | [[image:100520JS1|200px|none]] | + | [[image:100520JS1.JPG|200px|none]] |
| - | ===May 25 | + | |
| + | ==<font color="white">May 25/2010== | ||
(in the lab:JV, HB, AV)<br> | (in the lab:JV, HB, AV)<br> | ||
| Line 722: | Line 881: | ||
<b>NOTE</b> (June 1/2010; AS): Likely that the problem here was the method used to heat shock the cells. Cells were heat shocked in a heat plate, with incomplete contact of the heating surface, causing inefficient transfer of heat to cells for shock and movement of DNA into competent cells. | <b>NOTE</b> (June 1/2010; AS): Likely that the problem here was the method used to heat shock the cells. Cells were heat shocked in a heat plate, with incomplete contact of the heating surface, causing inefficient transfer of heat to cells for shock and movement of DNA into competent cells. | ||
| - | ===May 25 | + | ==<font color="white">May 25/2010 Evening== |
(in the lab: AV, KG)<br> | (in the lab: AV, KG)<br> | ||
| Line 816: | Line 975: | ||
*T4 DNA ligase added to ligations at 8:37pm. | *T4 DNA ligase added to ligations at 8:37pm. | ||
*Incubated reactions overnight at room temperature | *Incubated reactions overnight at room temperature | ||
| - | *Inactived by heating for 10 minutes at 80<sup>o</sup>C at 10:00am | + | *Inactived by heating for 10 minutes at 80<sup>o</sup>C at 10:00am<br> |
| + | Analyze restrictions and ligations by electrophoresis on a 1% agarose gel, stained with ethidium bromide. Load order is as follow: | ||
| + | <table><table border="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Volume Rxn (µL)</b></td><td><b>Volume Dye (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>Restricted mms6 (B6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>2</td><td>Unrestricted mms6 (B6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>3</td><td>Restricted mms6 (A6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>4</td><td>Unrestricted mms6 (A6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>5</td><td>mms6 (B6)-dT ligation</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>6</td><td>mms6 (A6)-dT Ligation</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>7</td><td>Restricted dT (B1)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>8</td><td>Unrestricted dT (B1)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>9</td><td>Restricted xylE (C4)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>10</td><td>Unrestricted xylE (C4)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>11</td><td>Restricted xylE (B4)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>12</td><td>Unrestricted xylE (B4)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>13</td><td>xylE (C4)-dT Ligation</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>14</td><td>xylE (B4)-dT Ligation</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>15</td><td>1kb Ladder</td><td>2 (+8water)</td><td>2</td></tr> | ||
| + | <tr><td>16</td><td>Empty</td><td></td><td></td></tr> | ||
| + | <tr><td>17</td><td>Empty</td><td></td><td></td></tr> | ||
| + | <tr><td>18</td><td>Empty</td><td></td><td></td></tr> | ||
| + | <tr><td>19</td><td>Empty</td><td></td><td></td></tr> | ||
| + | <tr><td>20</td><td>Empty</td><td></td><td></td></tr></table> | ||
| + | Gel was run at 100V for 2 hours<br> | ||
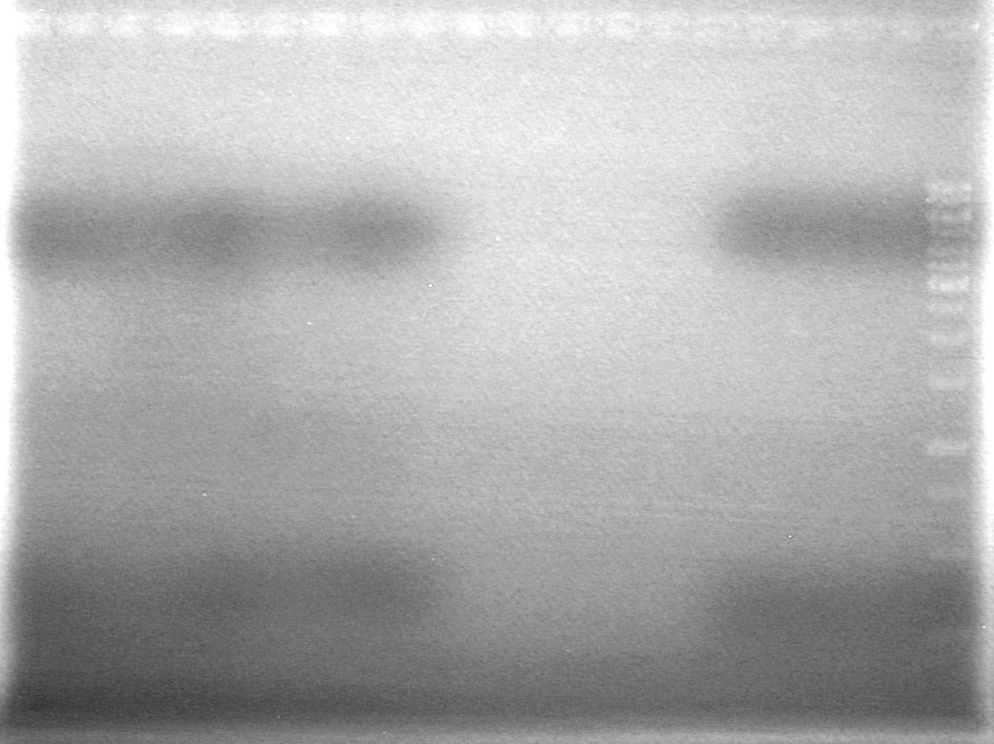
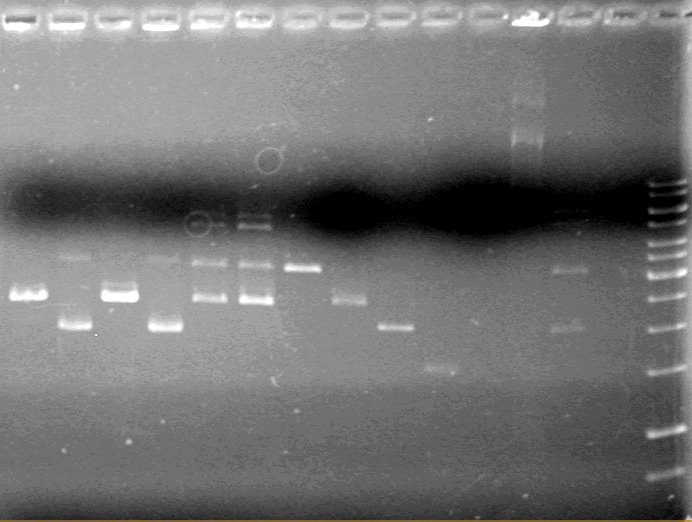
| + | <b>Results:</b><br> | ||
| + | [[image:100525 JV XYLE MMS6 Ligation.jpg|200px|none]] | ||
| + | |||
| + | <b>Objective:</b>Grow overnight cultures of cells for pDNA mini-prep tomorrow.<br> | ||
| + | <b>Method:</b><br> | ||
| + | DH5α cells from -80<sup>o</sup>C freezer containing the below genes were inoculated into 5mL LB liquid broth containing 100µg/mL of ampicillin and subsequently incubated at 37<sup>o</sup>C with shaking (at 300RPM) overnight (beginning at 7:30pm)<br> | ||
| + | Cells with the following gene products were retrieved from the -80<sup>o</sup>C freezer: | ||
| + | <table><table border="3"> | ||
| + | <tr><td><b>Common Name</b></td><td><b>Box</b></td><td><b>Location</b></td><td><b>Result</b></td></tr> | ||
| + | <tr><td>Fusion CEYFP</td><td>2007</td><td>H5,I5,J5</td><td>All grew</td></tr> | ||
| + | <tr><td>CEYFP</td><td>2007</td><td>G6,H6</td><td>All grew</td></tr> | ||
| + | <tr><td>NEYFP</td><td>2007</td><td>I6,J6</td><td>All grew</td></tr> | ||
| + | <tr><td>Arg N-term</td><td>2007</td><td>I7,J7</td><td>None grew</td></tr> | ||
| + | <tr><td>Arg C-term</td><td>2007</td><td>J8</td><td>All grew</td></tr> | ||
| + | <tr><td>ECFP</td><td>2009</td><td>A3,B3,C3</td><td>All grew</td></tr> | ||
| + | <tr><td>EYFP</td><td>2009</td><td>A6,B6,C6</td><td>All grew</td></tr> | ||
| + | <tr><td>TetR Inverter</td><td>2009</td><td>A9,B9</td><td>None grew</td></tr> | ||
| + | <tr><td>pBad-TetR</td><td>2007/2009</td><td>G8/C9</td><td>All grew</td></tr></table> | ||
| + | Removed at 9:30pm<br> | ||
| + | |||
| + | ==<font color="white">May 26/2010== | ||
| + | (In the lab: JV)<br> | ||
| + | <b>Objective:</b> Purify plasmid DNA (via mini-prep) of the cells grown last night.<br> | ||
| + | <b>Method:</b> Performed [[Team:Lethbridge/Notebook/Protocols|boiling lysis miniprep]] as described in protocols section on cells containing the following genes:<br> | ||
| + | * Fusion CEYFP (H5 - 2007) | ||
| + | * Fusion CEYFP (J5 - 2007) | ||
| + | * NEYFP (J6 - 2007) | ||
| + | * NEYFP (I6 - 2007) | ||
| + | * Fusion CEYFP (I5 - 2007) | ||
| + | * CEYFP (G6 - 2007) | ||
| + | * pBad-TetR (G8 - 2007) | ||
| + | * CEYFP (H6 - 2007) | ||
| + | * EYFP (A6 - 2009) | ||
| + | * ECFP (A3 - 2009) | ||
| + | * pBad-TetR (C9 - 2009) | ||
| + | * ECFP (C3 - 2009) | ||
| + | * EYFP (B6 - 2009) | ||
| + | * EYFP (C6 - 2009) | ||
| + | * ECFP (B3 - 2009) | ||
| + | <b>Results:</b> Will restrict and run agarose gels tomorrow.<br> | ||
| + | |||
| + | ==<font color="white">May 27/2010== | ||
| + | (In the lab: JV, AV)<br> | ||
| + | |||
| + | <b>Objective:</b>Restrict and run agarose gels of plasmids 'minipreped' yesterday from the 2007 and 2008 glycerol stock boxes.<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | Master Mix for Restriction Reactions: | ||
| + | <table><table border="3"> | ||
| + | <tr><td>Ingredient</td><td>Volume/tube (µL)</td><td>(Volume/tube(µL)) X 16</td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O</td><td>15.75</td><td>252</td></tr> | ||
| + | <tr><td>Orange</td><td>2</td><td>32</td></tr> | ||
| + | <tr><td>EcoRI</td><td>0.25</td><td>4</td></tr></table><br> | ||
| + | Master Mix for unrestricted DNA: | ||
| + | <table><table border="3"> | ||
| + | <tr><td>Ingredient</td><td>Volume/tube (µL)</td><td>(Volume/tube(µL)) X 16</td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O</td><td>16</td><td>256</td></tr> | ||
| + | <tr><td>Orange</td><td>2</td><td>32</td></tr></table><br> | ||
| + | |||
| + | 18µL of restriction master mix was added to 2(µL) of each plasmid.<br> | ||
| + | 18µL of unrestricted master mix was added to 2(µL) of each plasmid.<br> | ||
| + | 18µL of Milli-Q H<sub>2</sub>O was added to 2(µL) of orange buffer as a buffer control.<br> | ||
| + | Reactions were carried out for 1 hour at 37<sup>o</sup>C.<br> | ||
| + | Analyzed on a 1% agarose gel. <br> | ||
| + | |||
| + | <b>Gel 1</b><br> | ||
| + | <table><table border="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Volume Rxn (µL)</b></td><td><b>Volume Dye (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kb Ladder<sup>†</sup></td><td>2</td><td>2</td></tr> | ||
| + | <tr><td>2</td><td>Buffer Control</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>3</td><td>CEYFP (H6) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>4</td><td>CEYFP (H6) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>5</td><td>CEYFP (G6) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>6</td><td>CEYFP (G6) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>7</td><td>EYFP (C6) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>8</td><td>EYFP (C6) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>9</td><td>Fusion CEYFP (I5) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>10</td><td>Fusion CEYFP (I5) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>11</td><td>EYFP (C3) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>12</td><td>EYFP (C3) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>13</td><td>pBad-TetR (C9) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>14</td><td>pBad-TetR (C9) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>15</td><td>ECFP (A3) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>16</td><td>ECFP (A3) Unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>17</td><td>Fusion CEYFP (H5) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>18</td><td>Fusion CEYFP (H5) unrestricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>19</td><td>pBad-TetR (G8) Restricted</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>20</td><td>pBad-TetR (G8) unrestricted</td><td>10</td><td>10</td></tr></table> | ||
| + | † Also added 8µL of water to this mix<br> | ||
| + | |||
| + | <b>Gel 2</b> | ||
| + | <table><table border="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Volume Rxn (µL)</b></td><td><b>Volume Dye (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kb Ladder<sup>†</sup></td><td>2</td><td>2</td></tr> | ||
| + | <tr><td>2</td><td>Restricted Fusion CEYFP (J5)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>3</td><td>Unrestriced Fusion CEYFP</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>4</td><td>Restricted ECFP (B3)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>5</td><td>Unrestricted ECFP (B3)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>6</td><td>Restricted EYFP (B6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>7</td><td>Unrestricted EYFP (B6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>8</td><td>Restricted NEYFP (I6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>9</td><td>Unrestricted NEYFP (I6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>10</td><td>Restricted EYFP (A6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>11</td><td>Unrestricted EYFP (A6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>12</td><td>Restricted EYFP (J6)</td><td>10</td><td>10</td></tr> | ||
| + | <tr><td>13</td><td>Unrestricted EYFP (J6)</td><td>10</td><td>10</td></tr></table> | ||
| + | † Also added 8µL of water to this mix<br><br> | ||
| + | Ran both gels at 100V for 2 hours.<br> | ||
| + | <b>Results:</b><br> | ||
| + | Gel 1<br> | ||
| + | [[image:100527 AV Plasmid Test 1.jpg|200px|none]]<br> | ||
| + | Gel 2<br> | ||
| + | [[image:100527 JV Plasmid Test 1.jpg|200px|none]]<br> | ||
| + | <b>Conclusion:</b> To be concluded....<br><br> | ||
| + | |||
| + | <b>Objective:</b> Transform DH5α cells with plasmids containing ligation products from May 25/2010.<br> | ||
| + | <b>Method:</b> | ||
| + | Transform, using the [[Team:Lethbridge/Notebook/Protocols|competent cell transformation]] protocol, the ligation products with the most intense banding from each of the following ligation reactions:<br> | ||
| + | *mms6 + dT (B6 + A6)<br> | ||
| + | *xylE + dT (B4 + C4)<br> | ||
| + | Incubate at 37<sup>o</sup>C overnight.<br> | ||
| + | <b>Results:</b><br> | ||
| + | No colonies grew, even on positive control plate.<br> | ||
| + | ===May 31/2010=== | ||
| + | <b>Objective:</b> Transform part BBa_J33204 (Ribosomal Binding Site + xylE gene) into DH5α cells.<br> | ||
| + | <b>Method:</b><br> | ||
| + | Obtained part BBa_J33204 from 2010 iGEM Spring Distribution Kit Plate 1, well 7P.<br> | ||
| + | Transformed using [[Team:Lethbridge/Notebook/Protocols|competent cell transformation]] protocol, along with negative control (milliQ H<sub>2</sub>O water) and positive control (pUC19 plasmid). | ||
| + | Plated 100µL and 50µL on separate pre-warmed LB agar plates containing 100µg/mL ampicillin.<br> | ||
| + | Added 250µL of SOC media and incubated for <u>90 minutes</u> at 37<sup>o</sup>. <br> | ||
| + | <b>Results:</b><br> | ||
| + | Obtained the following number of colonies on each plate: | ||
| + | *BBa_J33204 50µL - 13 colonies<br> | ||
| + | *BBa_J33204 100µL - 18 colonies<br> | ||
| + | *pUC19 - 9 colonies<br> | ||
| + | <b>Other lab activities:</b><br> | ||
| + | *Inoculated 5mL of LB liquid broth (Amp<sup>+</sup> with cells containing Bba_J33204 picked from colony off transformation plate and incubated at 37<sup>o</sup>C with shaking overnight.<br><br> | ||
| + | AV quantified each pDNA stock in the [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids box]] by measuring the A<sub>260</sub> of a 1:100 dilution of the pDNA samples in a UV cuvette. Results are posted on the [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids]] page.<br> | ||
| + | |||
| + | ==<font color="white">May 31/2010 - Evening== | ||
| + | <b>Objective:</b> Transform recent ligation reactions that didn't work the first time around.<br> | ||
| + | <b>Method:</b> Use [[Team:Lethbridge/Notebook/Protocols|competent cell transformation]] protocol to transform the following ligation products:<br> | ||
| + | *pLacI + sRBS<br> | ||
| + | *pLacI + sRBS-Lum-dT (times 2)<br> | ||
| + | *mms6 (B6) + dT<br> | ||
| + | *mms6 (A6) + dT<br> | ||
| + | *xylE (C4) + dT<br> | ||
| + | *xylE (B4) + dT<br> | ||
| + | Plated all cells; spun down media so cells were pelleted and removed 200uL of cell-free media. Re-suspended pelleted cells in remaining media, and plated onto ampicillin plates.<br> | ||
| + | <b>Results:</b><br> | ||
| + | No growth on plates EXCEPT: | ||
| + | *Positive control (pUC19) - 9 colonies <br> | ||
| + | *mms6 (B6) + dT - 1 colonies <br><br> | ||
| + | <br> | ||
 "
"