Team:Lethbridge/Notebook/Lab Work/June
From 2010.igem.org
Liszabruder (Talk | contribs) |
Adam.smith4 (Talk | contribs) (→June 28/2010) |
||
| (6 intermediate revisions not shown) | |||
| Line 57: | Line 57: | ||
</th> | </th> | ||
| - | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook"> | + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> |
<img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
</a> | </a> | ||
| Line 105: | Line 105: | ||
<body> | <body> | ||
<center> | <center> | ||
| - | <table border="0" width=" | + | <table border="0" width="28%" style="background-color:#000000"> |
<tr> | <tr> | ||
| Line 112: | Line 112: | ||
<div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
<div class="miniContainer"> | <div class="miniContainer"> | ||
| - | + | ||
| - | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook"> | + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> |
| - | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width=" | + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> |
</a> | </a> | ||
</th> | </th> | ||
| Line 120: | Line 120: | ||
<th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols"> | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols"> | ||
<img src="https://static.igem.org/mediawiki/2010/9/91/UofLprotocolsbutton.jpg" width="60"/> | <img src="https://static.igem.org/mediawiki/2010/9/91/UofLprotocolsbutton.jpg" width="60"/> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</a> | </a> | ||
</th> | </th> | ||
| Line 132: | Line 127: | ||
</a> | </a> | ||
</th> | </th> | ||
| + | |||
<th> | <th> | ||
<div class="miniBar"> | <div class="miniBar"> | ||
| Line 154: | Line 150: | ||
<body> | <body> | ||
<center> | <center> | ||
| - | <table border="0" width=" | + | <table border="0" width="50%" style="background-color:#000000"> |
<tr> | <tr> | ||
| Line 193: | Line 189: | ||
</th> | </th> | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<tr> | <tr> | ||
| Line 1,255: | Line 1,248: | ||
*all Phusion tubes are <font color ="Blue">blue</font><br> | *all Phusion tubes are <font color ="Blue">blue</font><br> | ||
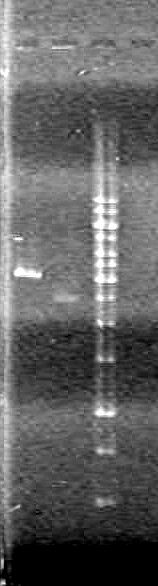
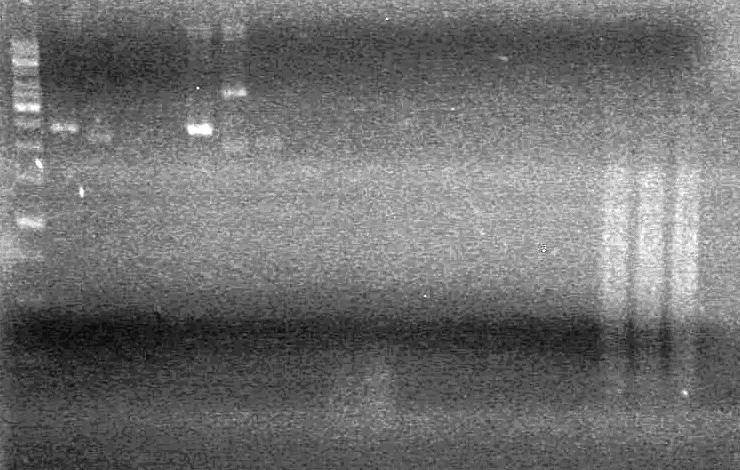
| - | <b>Results:</b> | + | <b>Results:</b> |
| + | <font color="red">IMAGE!!!</font> | ||
| Line 1,394: | Line 1,388: | ||
<tr><td>33</td><td>1kb ladder</td><td>0.5 ladder + 2 Dye (6X) + 9.5 Milli-Q H<sub>2</sub>O</td></tr> | <tr><td>33</td><td>1kb ladder</td><td>0.5 ladder + 2 Dye (6X) + 9.5 Milli-Q H<sub>2</sub>O</td></tr> | ||
</table><br> | </table><br> | ||
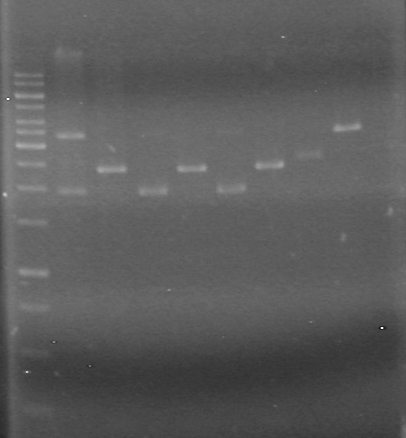
| - | + | <b>Results:</b> [[image:Lethbridge_100624PCR.JPG|200px]] | |
| - | < | + | |
==<font color="white">June 28/2010== | ==<font color="white">June 28/2010== | ||
| Line 1,460: | Line 1,453: | ||
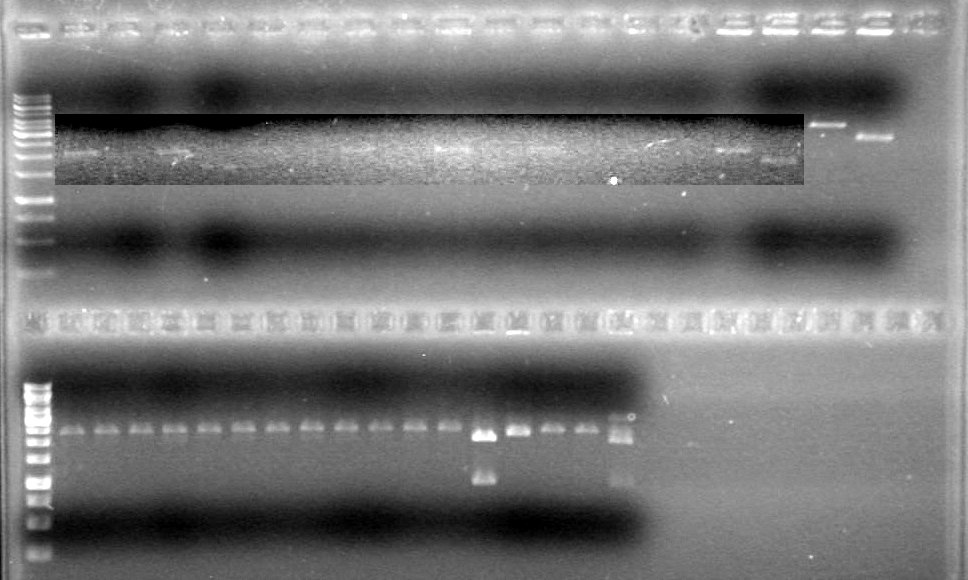
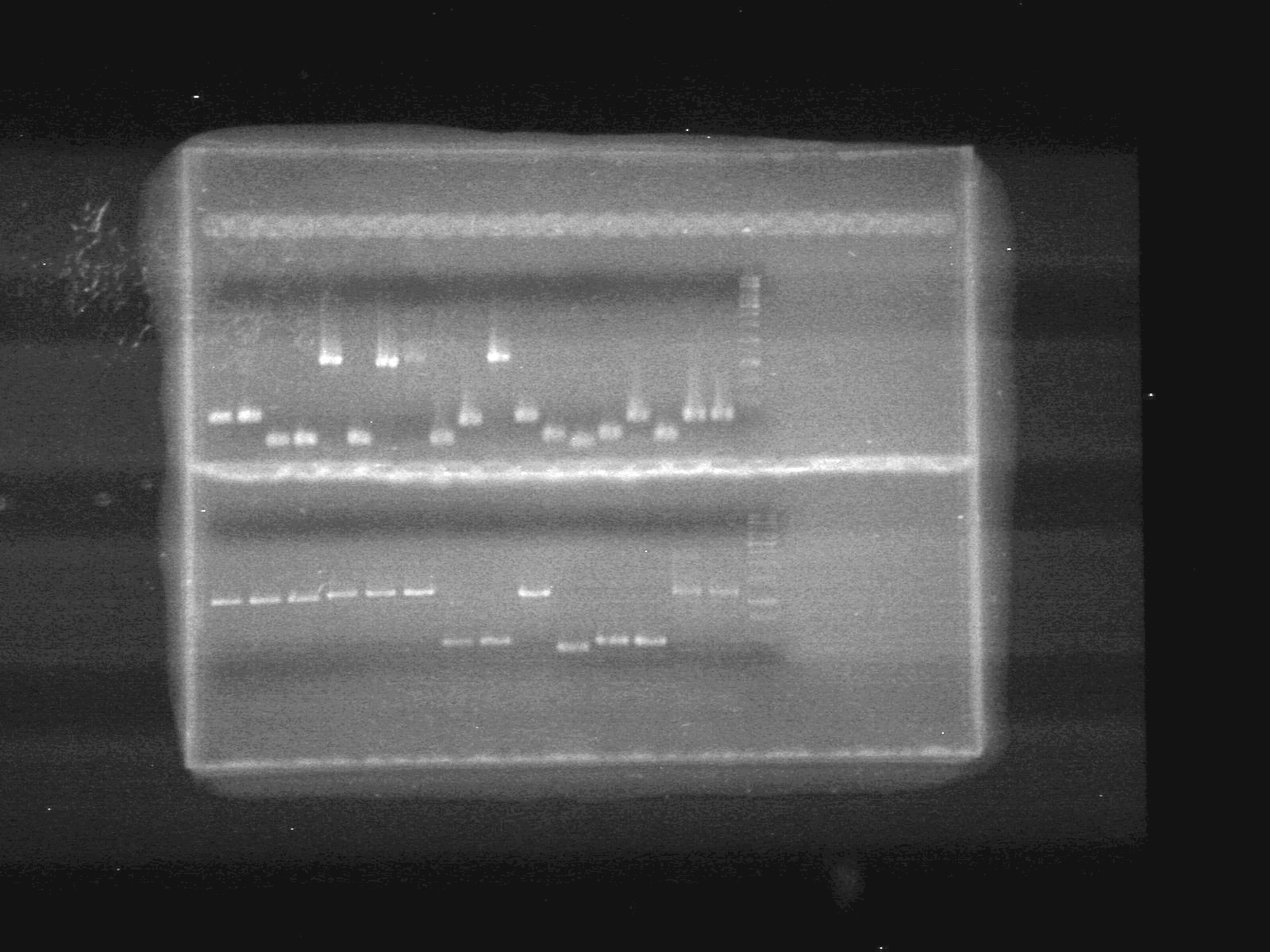
<b>Results:</b>pET28(a) bands are all smeared, and are not useful from inserting parts.<br> | <b>Results:</b>pET28(a) bands are all smeared, and are not useful from inserting parts.<br> | ||
| - | + | [[image:Lethbridge_100628AV (2).JPG|200px]]<br> | |
| - | + | ||
==<font color="white">June 30/2010== | ==<font color="white">June 30/2010== | ||
 "
"