Team:LMU-Munich/Jump-or-die/Schedule
From 2010.igem.org
(New page: {{:Team:LMU-Munich/Templates/Page Header}} {{:Team:LMU-Munich/Templates/Page Footer}}) |
|||
| (30 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:LMU-Munich/Templates/Page Header}} | {{:Team:LMU-Munich/Templates/Page Header}} | ||
| + | |||
| + | [[image:Jumplogo.png|250px|right|ApoControl logo]] | ||
| + | |||
| + | |||
| + | ==Aim 1: DNA reproduction+PCR== | ||
| + | |||
| + | For PCR key see [[Team:LMU-Munich/Cut'N'survive/Schedule/PCR key | PCR key]] | ||
| + | |||
| + | <b>Colourcode for the primers: <font color="#FF0000">Annealing part</font>, <font color="#009933">mutagenised part</font>, <font color="#FF6600">other sequences integrated into primer</font></b> | ||
| + | |||
| + | |||
| + | <b>PCR1: replication of the Tet-inducible CMV promotor [X]</b> | ||
| + | [[Image:PCR1.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer1| 1TreF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer2| 2TreR]] | ||
| + | expected product size: 492 bp | ||
| + | |||
| + | |||
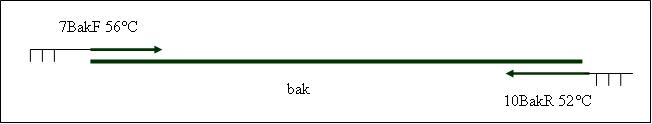
| + | <b>PCR4a: replication human bak with mutagenesis (Pst1) [X]</b> | ||
| + | [[Image:PCR4a.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer7| 7BakF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer8| 8BakMutPR]] | ||
| + | expected product size: 330 bp | ||
| + | |||
| + | |||
| + | <b>PCR4b: replication human bak with mutagenesis (Pst1) [X]</b> | ||
| + | [[Image:PCR4b.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer9| 9BakMutPF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer10| 10BakR]] | ||
| + | expected product size: 376 bp | ||
| + | |||
| + | |||
| + | <b>PCR5: joining PCR of human bak [X]</b> | ||
| + | [[Image:PCR5.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer7| 7BakF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer10| 10BakR]] | ||
| + | expected product size: 688 bp | ||
| + | |||
| + | |||
| + | <b>PCR6: replication of the SV40-polyadenylation site [X]</b> | ||
| + | [[Image:PCR6.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer11| 11PAF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer12| 12PAR]] | ||
| + | expected product size: 237 bp | ||
| + | |||
| + | |||
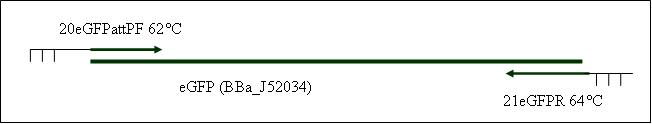
| + | <b>PCR9: replication of eGFP with attP in primer [X]</b> | ||
| + | [[Image:PCR9.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer20| 20eGFPattPF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer21| 21eGFPR]] | ||
| + | expected product size: 808 bp | ||
| + | |||
| + | |||
| + | <b>PCR10: Replikation of PhiC31o [X]</b> | ||
| + | [[Image:PCR10.jpg]] | ||
| + | |||
| + | Primer used:[[Team:LMU-Munich/Cut'N'survive/Schedule/Primer22| 22PC31oF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer23| 23PC31oR]] | ||
| + | expected product size: 1888 bp | ||
| + | |||
| + | <b>Note: for attB two Primers ([[Team:LMU-Munich/Cut'N'survive/Schedule/Primer18| 18attBF]][[Team:LMU-Munich/Cut'N'survive/Schedule/Primer19| 19attBR]]) used.</b> | ||
| + | |||
| + | ==Aim 2: Inserting PCR Products in pSB1C3 and verifying Sequence == | ||
| + | |||
| + | PCR1 (BBa_K368001): Insertion [ ] -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenzPCR1| Sequence of PCR1 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | PCR5 (BBa_K368017): Insertion [ ] -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenzPCR5| Sequence of PCR5 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | PCR6 (BBa_K368018): Insertion [ ] -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenzPCR6| Sequence of PCR6 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | PCR9 (BBa_K368021): Insertion [ ] -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenzPCR9| Sequence of PCR9 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | PCR10 (BBa_K368022): Insertion [ ] -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenzPCR10| Sequence of PCR10 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | ==Aim 3: Assembling Biobricks== | ||
| + | |||
| + | We are using the [[Team:LMU-Munich/Notebook/Protocols/13_3A_Method_for_Biobrick_assembly|3A System]] to assemble Biobricks. | ||
| + | |||
| + | Assembly of BioBricks: | ||
| + | |||
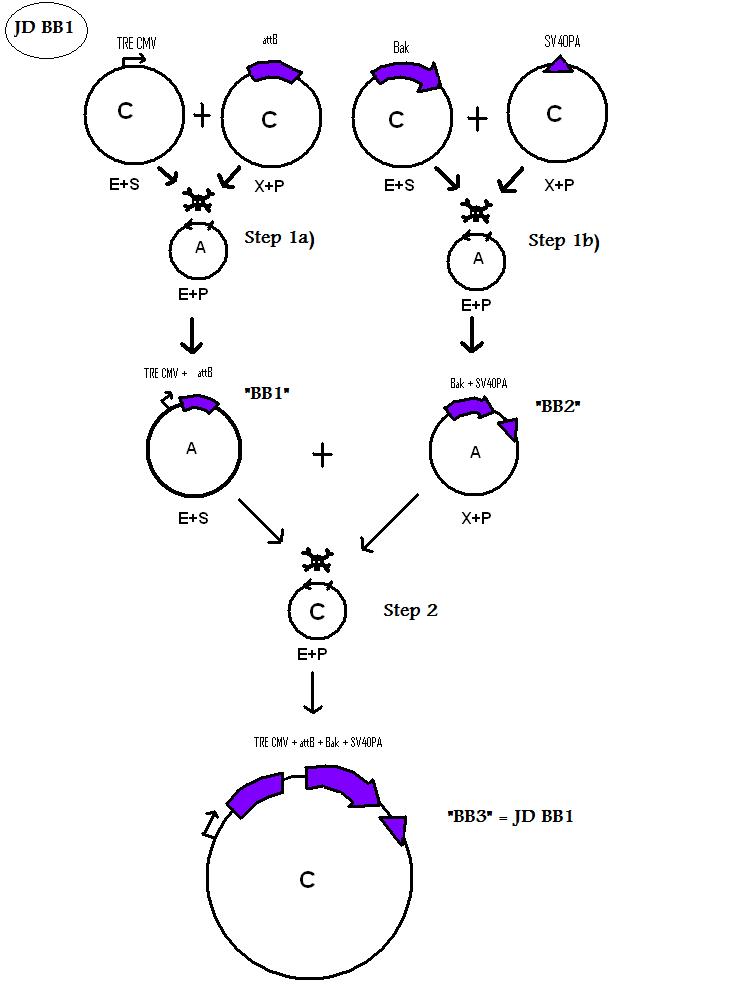
| + | * BB1 ( BBa_K368024): Assembly [ ]; prevTRE CMV + attB site | ||
| + | |||
| + | -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenceBB1| Sequence of BB1 from sequencing]]: confirmed [ ] | ||
| + | |||
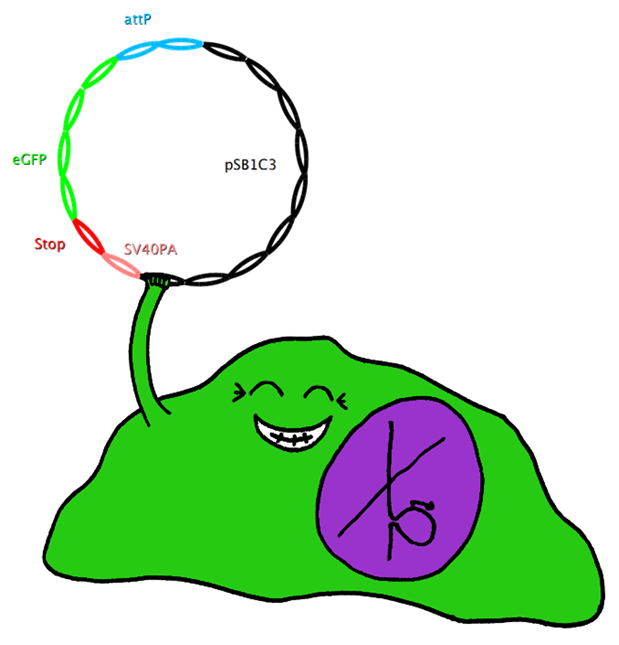
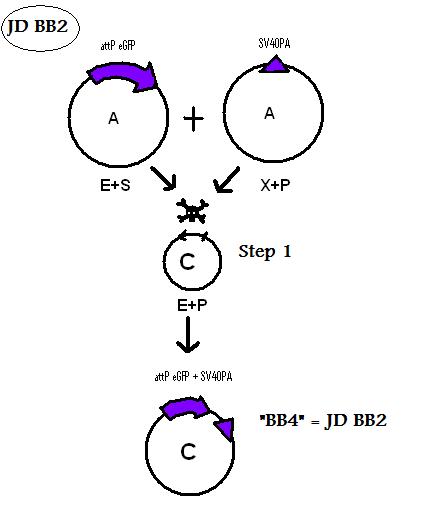
| + | * BB2 ( BBa_K368002): Assembly [ ]; Human Bak + SV40PA | ||
| + | |||
| + | -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenceBB2| Sequence of BB2 from sequencing]]: confirmed [ ] | ||
| + | |||
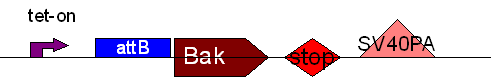
| + | * BB3 ( BBa_K368003): Assembly [ ]; prevTRE CMV+ attB site + Human Bak + SV40PA | ||
| + | |||
| + | -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenceBB3| Sequence of BB3 from sequencing]]: confirmed [ ] | ||
| + | |||
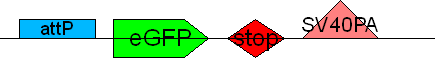
| + | * BB4 ( BBa_K368004): Assembly [X]; eGFP + SV40PA | ||
| + | |||
| + | -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenceBB4| Sequence of BB4 from sequencing]]: confirmed [X] | ||
| + | |||
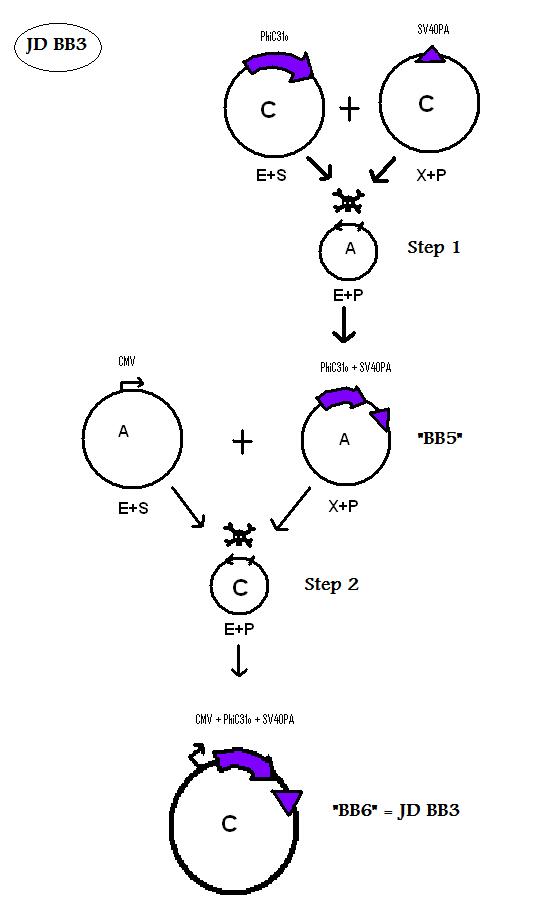
| + | * BB5 ( BBa_K368005): Assembly [ ]; PhiC31o + SV40PA | ||
| + | |||
| + | -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenceBB5| Sequence of BB5 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | * BB6 ( BBa_K368006): Assembly [ ]; CMV-promoter + PhiC31o + SV40PA | ||
| + | |||
| + | -> [[Team:LMU-Munich/Jump-or-die/Schedule/SequenceBB6| Sequence of BB6 from sequencing]]: confirmed [ ] | ||
| + | |||
| + | |||
| + | <b> Assembling Construct 1</b> | ||
| + | |||
| + | [[Image: J1S.jpg]] | ||
| + | |||
| + | <b> Assembling Construct 2</b> | ||
| + | |||
| + | [[Image: J2S.jpg]] | ||
| + | |||
| + | <b> Assembling Construct 3</b> | ||
| + | |||
| + | [[Image: J3S.jpg]] | ||
| + | |||
| + | ==Aim 4: Testing products== | ||
| + | |||
| + | === Construct 1 === | ||
| + | |||
| + | |||
| + | [[Image: Konstruktjump1.PNG | 400pxs | Jump construct 1]] | ||
| + | |||
| + | - Transform into HeLa cells to see if they survive. | ||
| + | |||
| + | -> Check the leakiness of tet-on-promoter | ||
| + | |||
| + | - Induce tet-on-promoter to see, if cells die. | ||
| + | |||
| + | -> Check if construct 1 is working | ||
| + | |||
| + | === Construct 2 === | ||
| + | |||
| + | [[Image: Konstruktjump2.PNG | 400pxs | Jump construct 2]] | ||
| + | |||
| + | - Sequencing | ||
| + | |||
| + | === Construct 3 === | ||
| + | |||
| + | [[Image: Konstruktjump3.PNG | 400pxs | Jump construct 3]] | ||
| + | |||
| + | - Transform into HeLa cells | ||
| + | |||
| + | - Western Blot or Proteinchromatography | ||
| + | |||
| + | -> Check if PhiC31o is read off | ||
| + | |||
| + | ==Aim 5: Testing system== | ||
| + | |||
| + | |||
| + | [[image:Jumplogo.png|200px|center|ApoControl logo]] | ||
| + | |||
| + | |||
{{:Team:LMU-Munich/Templates/Page Footer}} | {{:Team:LMU-Munich/Templates/Page Footer}} | ||
Latest revision as of 14:44, 25 October 2010
For PCR key see PCR key
Colourcode for the primers: Annealing part, mutagenised part, other sequences integrated into primer
Primer used: 1TreF 2TreR
expected product size: 492 bp
Primer used: 7BakF 8BakMutPR
expected product size: 330 bp
Primer used: 9BakMutPF 10BakR
expected product size: 376 bp
Primer used: 7BakF 10BakR
expected product size: 688 bp
Primer used: 11PAF 12PAR
expected product size: 237 bp
Primer used: 20eGFPattPF 21eGFPR
expected product size: 808 bp
Primer used: 22PC31oF 23PC31oR
expected product size: 1888 bp
Note: for attB two Primers ( 18attBF 19attBR) used.
PCR1 (BBa_K368001): Insertion [ ] -> Sequence of PCR1 from sequencing: confirmed [ ]
PCR5 (BBa_K368017): Insertion [ ] -> Sequence of PCR5 from sequencing: confirmed [ ]
PCR6 (BBa_K368018): Insertion [ ] -> Sequence of PCR6 from sequencing: confirmed [ ]
PCR9 (BBa_K368021): Insertion [ ] -> Sequence of PCR9 from sequencing: confirmed [ ]
PCR10 (BBa_K368022): Insertion [ ] -> Sequence of PCR10 from sequencing: confirmed [ ]
We are using the 3A System to assemble Biobricks.
Assembly of BioBricks:
-> Sequence of BB1 from sequencing: confirmed [ ]
-> Sequence of BB2 from sequencing: confirmed [ ]
-> Sequence of BB3 from sequencing: confirmed [ ]
-> Sequence of BB4 from sequencing: confirmed [X]
-> Sequence of BB5 from sequencing: confirmed [ ]
-> Sequence of BB6 from sequencing: confirmed [ ]
Assembling Construct 2
Assembling Construct 3
- Transform into HeLa cells to see if they survive.
-> Check the leakiness of tet-on-promoter
- Induce tet-on-promoter to see, if cells die.
-> Check if construct 1 is working
- Sequencing
- Transform into HeLa cells
- Western Blot or Proteinchromatography
-> Check if PhiC31o is read off


![]()
![]()







![]()
![]()

![]()
Contents
Aim 1: DNA reproduction+PCR
PCR1: replication of the Tet-inducible CMV promotor [X]

PCR4a: replication human bak with mutagenesis (Pst1) [X]
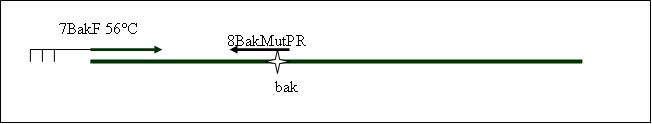
PCR4b: replication human bak with mutagenesis (Pst1) [X]
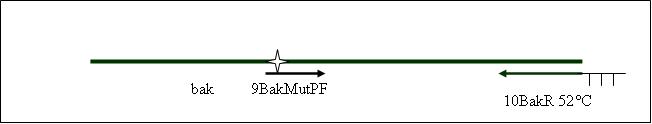
PCR5: joining PCR of human bak [X]
PCR6: replication of the SV40-polyadenylation site [X]

PCR9: replication of eGFP with attP in primer [X]
PCR10: Replikation of PhiC31o [X]

Aim 2: Inserting PCR Products in pSB1C3 and verifying Sequence
Aim 3: Assembling Biobricks
Assembling Construct 1
Aim 4: Testing products
Construct 1
Construct 2
Construct 3
Aim 5: Testing system
![]()
![]()

 "
"