Team:Johns Hopkins/Project
From 2010.igem.org
Contents |
Abstract
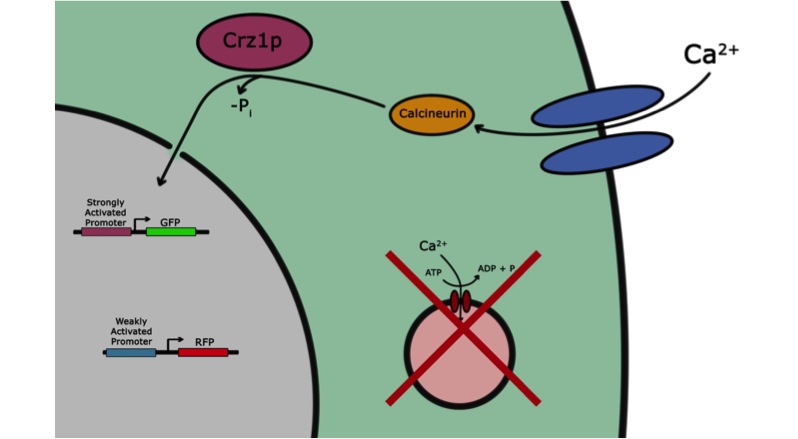
If the goal of iGEM and the Parts Registry is to take the messy world of genetic engineering and transform it into something like the standardized world of electrical engineering, it may be useful if electronic systems could directly interface with biological systems. Past iGEM projects have used chemical or optical stimuli to actuate transcriptional responses. Our project, however, seeks to add voltage sensitivity to Saccharomyces cerevisiae. Baker’s yeast was chosen because in some sense yeast have a system that responds to voltage input. With a voltage stimulus one can open the voltage-gated calcium channels of yeast, causing calcium ions to rush into the cytoplasm. This causes calcineurin to dephosphorylate Crz1, which enters the nucleus and binds various promoters. Our group presents a library of characterized Crz1-sensitive binding sites of both naturally-occurring and synthetic varieties that can be integrated into promoters. Genes downstream of these promoters are thus voltage-regulated in media containing calcium.
Aims
- Show that voltage can be used to stimulate a transcriptional response in S. cerevisiae.
- Develop a library of voltage-inducible promoters with differing voltage response curves.
- Determine the functional range of and optimized values for our system with respect to the following variables:
- Voltage applied
- Duration of voltage application
- Presence of vacuoles, yeast’s natural mode of intracellular Calcium control
- Develop an effective experimental apparatus to apply voltage and measure response.
Methods
Visualizing the Crz1 Transcription Factor
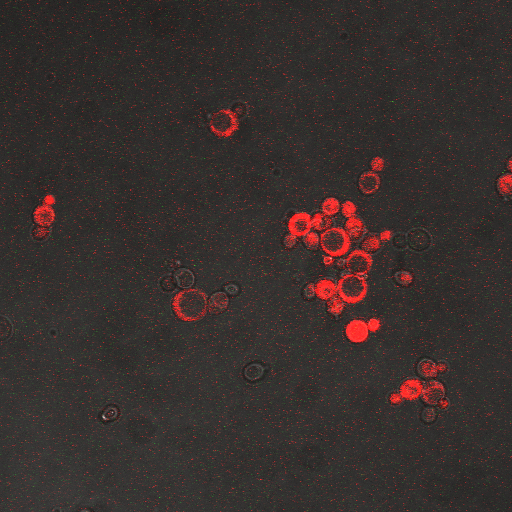
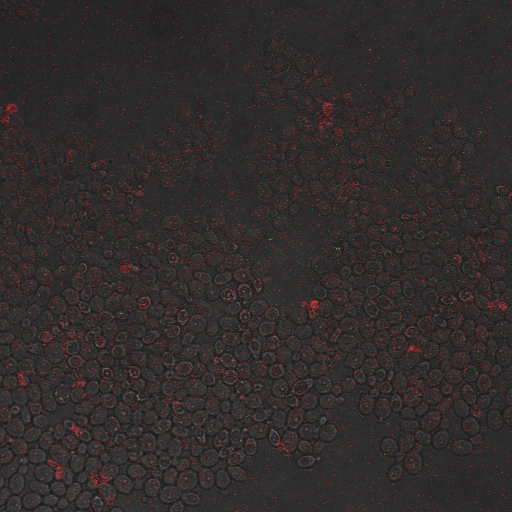
We want to characterize the electro stimulation voltage domain in which we see movement of the GFP tagged CRZ1 transcription factor into the nucleus. Crz1-GFP was grown and passed into two rows of a 96 well plate. The cells were then shocked every two hours at 10 Volts. The cells were passed to a glass plate where they were fixed and then imaged. GFP was observed to move in and out of the nucleus just 5 minutes after voltage stimulus. In a given image, some cells displayed GFP densely packed in the nucleus while other cells displayed GFP in the cytoplasm, but excluded from the nucleus. Here we see a confirmation of the oscillating behavior described by Elowitz et al (Elowitz 2008).
In this video we can see the GFP tagged CRZ1 cells displaying nuclear localization after shocking. Note that Crz1's oscillating movement into and out of the nucleus is not in phase throughout the cell population. GFP appears focussed in the nucleus in some cells, but in other cells GFP fills a donut-shaped area, leaving a void (the nucleus). Only a few cells actually exhibit a change in Crz1 localization during the course of the video.
Optimizing Parameters for the CDRE from the FKS2 Promoter
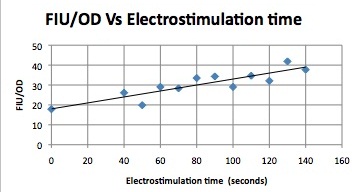
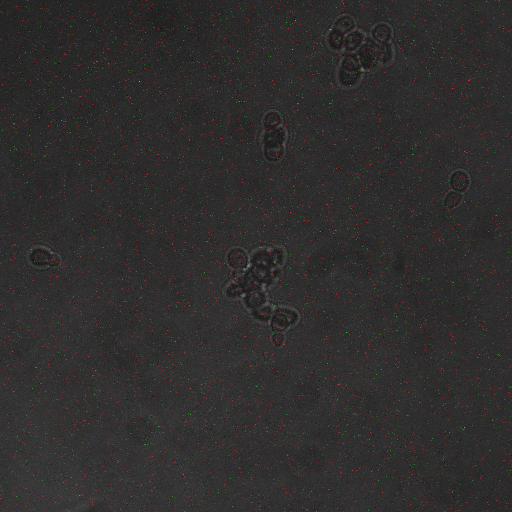
We want to find an optimum voltage amplitude and electro stimulation time for yeast containing the CDRE-RFP plasmid. Shocking at 10 Volts caused large amount of cell death, as such, it was necessary to find an optimal voltage to cause transcription, but not to damage our cells. CDRE-mCherry were grown and passed into 96 well plate. The electroporator was used to shock the cell with voltages from 2-10 Volts with an exposure time from 0-80 seconds. We find optimum electro stimulation voltage is 8V and we need at least 40 seconds of electrostimulation to see expression.
| 2s | 5s | 10s | 20s | 40s | 80s | |
|---|---|---|---|---|---|---|
| 10V | None | None | None | None | None | N/A |
| 8V | None | None | None | None | High | High |
| 6V | None | None | None | None | Moderate | High |
| 4V | None | None | None | None | Low | Low |
Further Honing FKS2 CDRE
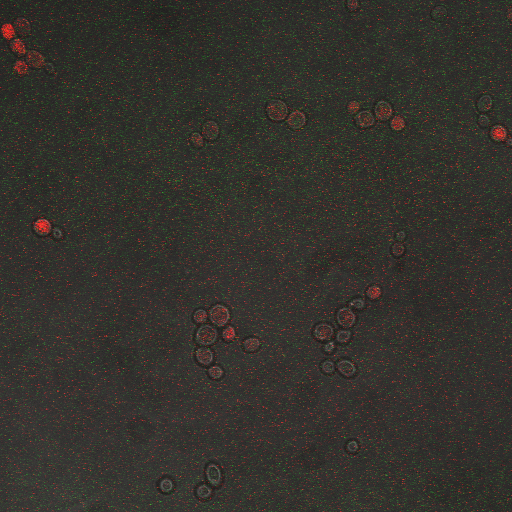
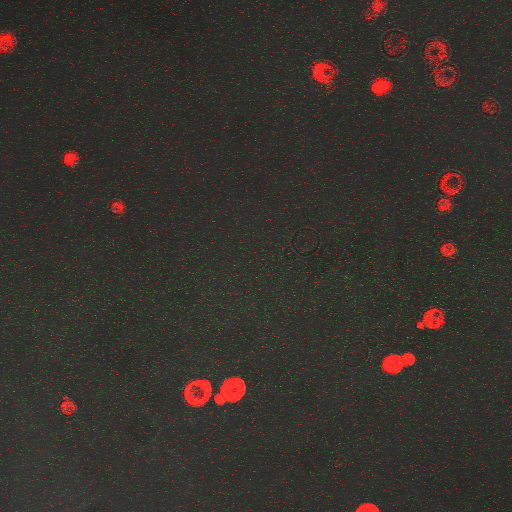
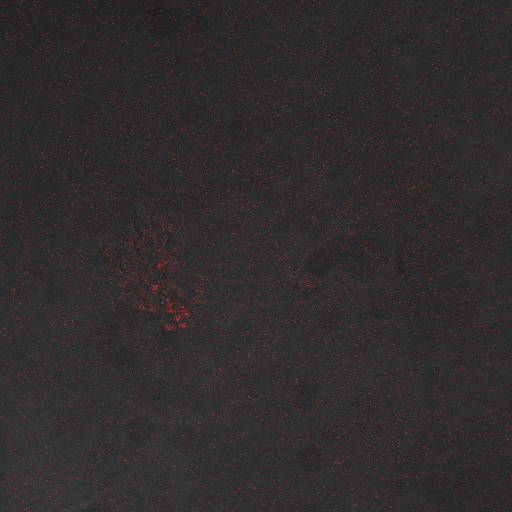
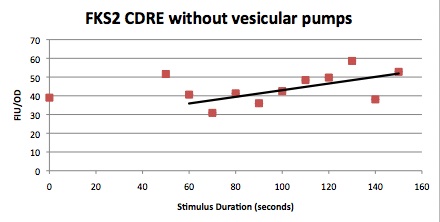
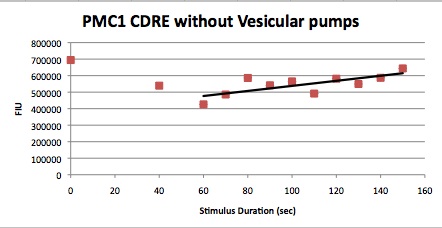
We want to hone in on the FSK2-CDRE systems region of activation in the domain of time of shocking. Also to compare yeast without PMC1 and VCX1 knocked out versus yeast with it not knocked out to determine effect of the knockout on the CDRE systems expression levels. From here we see a through characterization of the system that shows increase in expression of our reporter RFP with increase in electro stimulation time. We also see that there is much higher expression of the cells without vacuoles knocked out than in cells with vacuoles knocked out.
From the images above we can see that there is some constitutive expression of RFP in the FSK2 CDRE with vesicular pumps, but there is a very clear increase in expression of RFP with increase of electro stimulating time to 90sec. In the picture taken of the cells that were stimulated for 110 seconds, we see a large vesicle, which makes sense as the was a huge calcium influx which was relocated to the vesicle. This also explain why the cells are a lot healthier looking than the cells in which the vesicular pumps were knocked out.
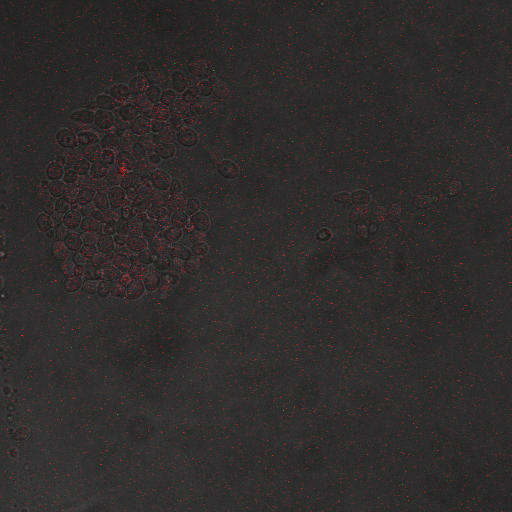
FKS2 CDRE and PMC1 CDRE in Vacuole Positive and Negative Yeast
Many cells were dying from the stress of voltage shocking, so FKS2 CDRE-mCherry was inserted into yeast that still had their calcium vacuoles. These cells were grown and passed into two rows of a 96 well plate and shocked at 2-10 volts with an exposure time of 0-80 seconds. We wanted to characterize the relationship between electrostimulation time and expression of florescent protein of the synthetic CDRE promoter sequence and the PMC1 promoter sequence. We also want to demonstrate the effect of knocking out the PMC1 and VCX1 genes in yeast. The data for both vacuole positive and vaculoe negative cells are shown below.All results are normalized against OD.
From these graphs we can see a clear linear relationship between the period of stimulation and the expression of RFP or GFP in the domain of stimulation time in which we tested. We also observed that there were significantly higher percentages of live cells in the cells in which the vacuoles were not knocked out, and the presence of the vacuoles did not have significant impact on the expression rates, aside from some constitutive expression being seen in the vacuole plus samples. The controls on all our experiments seem to indicate very high constitutive expression, but that is due to a combination of cell death due to stimulation, only a small fraction of cells being stimulated and background fluorescence and so it can be discounted.
Instrumentation
We tried several approaches to efficiently and accurately electrostimulate our cells without killing them:
- Microfluidic Chip
- Consists of 300 micron-wide channels with titanium and gold electrodes to electrically stimulate the cells.
- We have completed the fabrication of this device and are currently perfecting the fabrication technique to improve device quality and performance.
- High-throughput and high-precision possible.
- See our fabrication protocol here.
- A 8 Well plate with aluminum electrodes which had a brass backing. This did show us our first results, but due to the inaccuracy of the instrument we had to abandon its use.
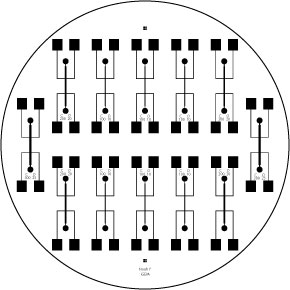
- Finally we have started using a gold plated coaxial 8 well electroporater to electrically stimulate the cells. It presents some problems as far as modeling the field effect on the cells goes, but it has shown excellent results and it allows us to do large scale experimentation using 96 well plates.
Future Research Plans
- Finish categorizing the synthetic CDREs using our high throughput electrostimulation device.
- We also plan to apply our optimized high throughput experimentation technique to a library of synthetic CDRE sequences we have developed to characterize their voltage activation domains. We hope this library might allow others to have a more fine-grained control over the voltage response in their cells.
- We hope to develop a genetic switch utilizing the repressible operator already present on FKS2.
- We noticed a lack of iGEM compatibility with the officially supported yeast chassis. We plan to develop an iGEM-compatible plasmid for yeast with a yeast-optimized origin of replication and markers. A shuttle vector would also be useful, enabling DNA to be stored or copied in bacteria and quickly transfered for testing in yeast. Our project would not be possible in a prokaryotic chassis. We feel that iGEM's apparent focus on E. coli may hinder the development of parts and systems that require eukaryotic organisms.
References
- Long Cai, Chiraj K. Dalal & Michael B. Elowitz. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature (2008).
- Yoshimoto et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol. Chem. (2002).
 "
"