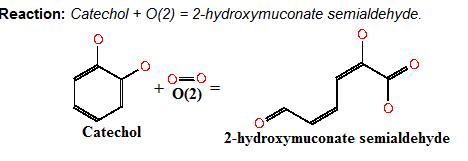Team:Imperial College London/Modules/Fast Response
From 2010.igem.org
| Line 129: | Line 129: | ||
Image taken from ftp://ftp.genome.jp/pub/kegg ; Where catechol is the colourless substrate converted by ring cleavage into 2 hydroxymuconate semialdehyde. | Image taken from ftp://ftp.genome.jp/pub/kegg ; Where catechol is the colourless substrate converted by ring cleavage into 2 hydroxymuconate semialdehyde. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
Revision as of 12:03, 24 October 2010
| Modules | Overview | Detection | Signaling | Fast Response |
| Our design consists of three modules; Detection, Signaling and a Fast Response, each of which can be exchanged with other systems. We used a combination of modelling and human practices to define our specifications. Take a look at the overview page to get a feel for the outline, then head to the full module pages to find out how we did it. | |
| Fast Response Module | Overview of the output |
|
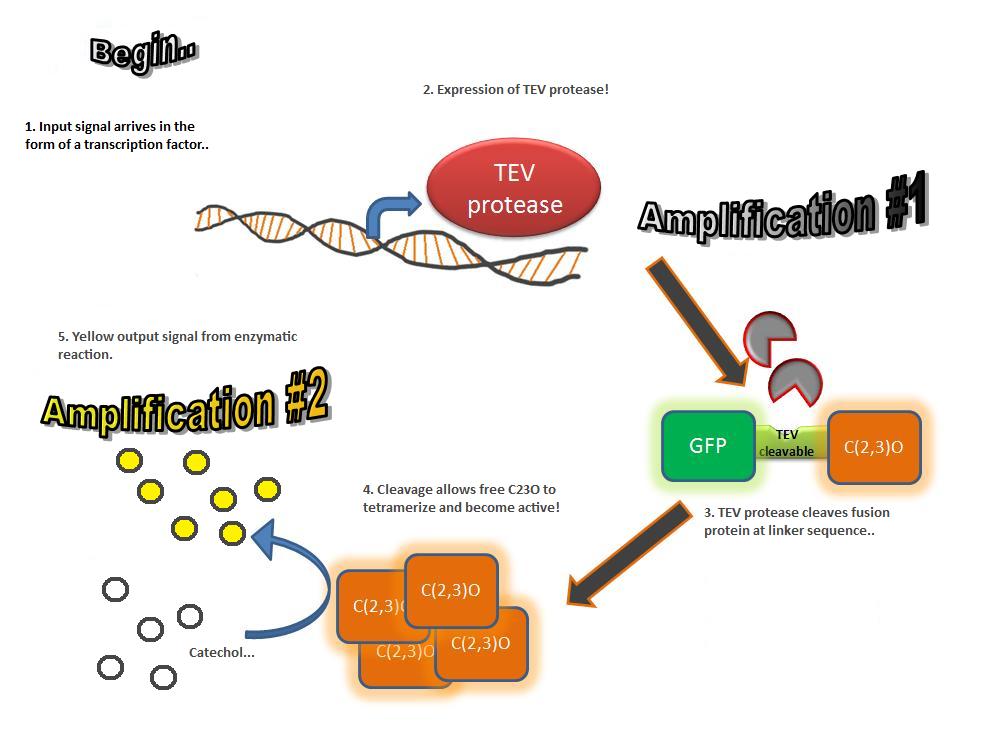
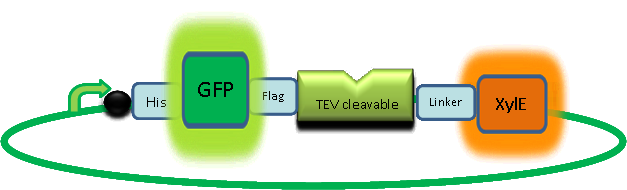
The third module of our bacterial detector consists of an fast response reporter module that upon input signal arrival it gives out a yellow optical signal output observable by naked eye. Traditional reporters in biochemical research usually rely on transcription-translation of a specific reporter molecule (ex. Green Fluorescence protein) which is time consuming and/or might involve sophisticate equipment for its detection. In this module we introduce a novel reporter system where expression of a small peptidase that acts on a pre-existing pool of substrate as well as a double amplification step should result in an output signal several orders of magnitude faster than traditional reporters. Below an quick overview of our system can be found, followed by a more in depth analysis of the system. |
| C230 and C230-GFP fusion |
|
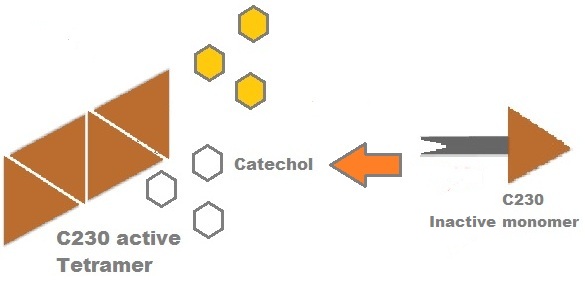
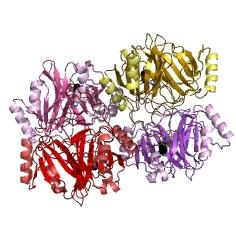
Catechol-2,3-dioxygenase (also known as C23O) is the protein product of XylE gene. It originates from Pseudomonas putida bacterium and the active protein is made of a homotetramer of monomers. The enzyme catalyses the conversion of a colourless substrate (catechol or substituted catechols) into a bright yellow product (2-hydroxymuconic semialdehyde) within seconds of substrate addition.
|
|
|
|
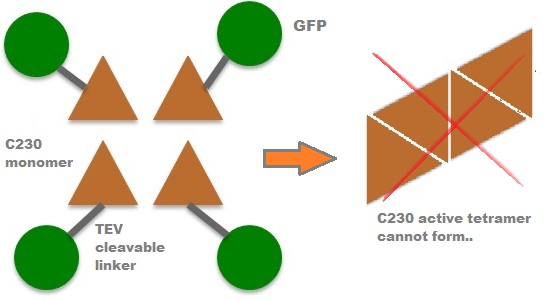
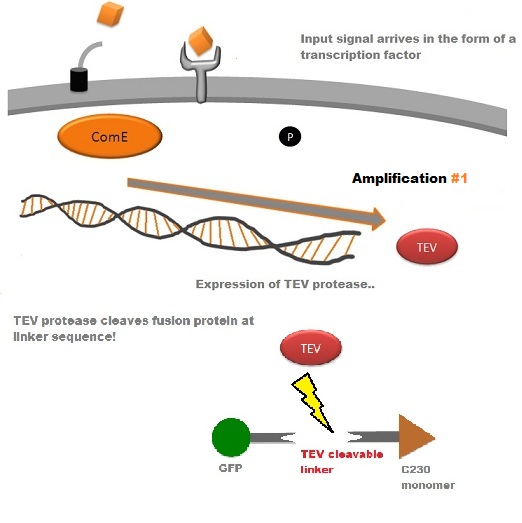
A very important feature of the fusion protein is its potential for induction of the system. A closer look at the fusion gene construct reveals the secret of the inducibility of our system. The GFP gene is fused to the XylE gene through a protein sequence that is susceptible to TEV protease. This protein sequence is recognized by the active site of the TEV protease so that when TEV is present in the cell, GFP is cleaved off Catechol-2,3-dioxygenase monomers. The free monomers are not longer stericly hindered by the GFP and are now able to associate with each other. Tetramerization of the monomers produces the active Catechol-2,3-dioxygenase enzyme, which in the presence of catechol substrate produces a yellow color. |
|
|
| TEV protease |
|
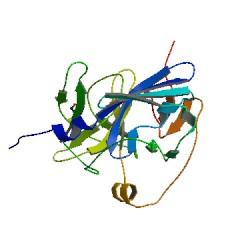
TEV protease is a natural polyprotein cutting viral protease. In our system ComE transcription factors bind to their native ComCDE promoter which was engineered as the promoter for TEV protease. This means that TEV protease is only transcribed and translated upon detection of the Schistosoma parasite.
Why TEV protease? This enzyme has been used extensively in protein engineering as it has a number of key qualities including a high specificity (ENYLFQG), and importantly for our system it is relatively tiny (242 aa) compared to other protease candidates available. Below is can be visualized as it has been deposited in the PDB with accession number 1Q31; TEV protease expression in E.Coli (and consequently B .subtilis) is made efficient by codon optimisation and inducing expression of additional tRNAs. |
| Fast < faster < Imperial College Turbo FAST |
|
So what are the key features that make our system a novel example of a fast response mechanism?
|
| C23O | Additional Material | ||||
|
After observing C23O in Pymol, and in addition information obtained from Kita et al which shows that the projecting loop is needed for dimerization and that this is very near to the N-terminus, we chose to engineer (by straightforward DNA synthesis) GFP plus linker (GGGSGGGS ENYLFQG) onto the N-termini of our C230 monomers. Below is the reaction performed by our systems enzyme:
Image taken from ftp://ftp.genome.jp/pub/kegg ; Where catechol is the colourless substrate converted by ring cleavage into 2 hydroxymuconate semialdehyde.
|
 "
"