This model consists of 5 parts that had to be developed:
- Identification of all active and relevant elements of the isolated part of the system.
- Identification of interactions between the identified elements.
- Identification of threshold concentration of auto inducing peptide (AIP) needed for activation of the receptor.
- Defining a control volume around the bacterial cell (after cleavage, the surface protein will float around in the extracellular environment).
- Determining the surface protein production to estimate the maximum abundance on the bacterial surface.
1. Elements of the system
- The surface protein consists of a cell wall binding domain, linker, AIP (Auto Inducing Peptide). It is expressed by constitutive gene expression. It was assumed that the bacteria would be fully grown before carrying out the detection so the cell wall would be covered with as many surface proteins as the cell can maintain.
- Schistosoma elastase (enzyme released by the parasite) cleaves the AIP from the cell wall binding domain at the linker site. In the laboratory we used TEV protease as we could not get hold of Schistosoma elastase, so the model was adjusted appropriately (TEV enzyme kinetic parameters were used).
- The ComD receptor is activated by a sufficiently high AIP concentration. Once activated, ComD signals into the cell to activate the colour response.
2. Interactions between elements
Apart from the proteins being expressed from genes, there was only one more chemical reaction identified in this part of the system. This is the cleavage of proteins, which is an enzymatic reaction:
- E+S ↔ ES → E+P
- Substrate (S) = Protein
- Enzyme (E) = TEV (Protease)
- Product (P) = Peptide
This enzymatic reaction can be rewritten as partial differential equations (PDEs), which is of similar form as the 1-step amplification model. However, most of the constants and initial concentrations are different.
3. Threshold concentration of AIP
The optimal peptide concentration required to activate ComD is 10 ng/ml [1]. This is the threshold value for ComD activation. However, the minimum concentration of peptide to give a detectable activation is 0.5ng/ml.
The threshold for the minimal activation of the receptor is cth=4.4658×10-9 mol/L. Click on the button below to uncover the calculations.
Converting 10 ng/ml to 4.4658×10-9 mol/L
- The mass of a peptide is 2.24kDa = 3.7184×10-21g.
- The number of molecules in one ml is 10ng/3.7184×10-21g = 2.6893×1012. In a litre, the number of molecules is 2.6893×1015molecules/L.
- Dividing this value by Avogadro's constant gives the threshold concentration of cth=4.4658×10-9 mol/L.
- The threshold for minimal activation of receptor is 2.2329×10-10 mol/L.
4. Control volume selection
Note that this enzymatic reaction is modelled outside the cell. Hence, it is important to take into account the cell boundaries. It is worth considering whether diffusion or fluid movements will play a significant role.
Initially, we defined a control volume assuming that bacteria would grow in close colonies on the plate.
This control volume is considered to be wrong, but the details were kept for reference.
Initial Choice of Control Volume
Control volume initial choice
This control volume is considered to be wrong by us, but the details were kept for the reference.
The control volume:
The inner boundary is determined by the bacterial cell (proteins after being displayed and cleaved cannot diffuse back into bacterium). The outer boundary is more time scale dependent. We have assumed that after mass cleavage of the display-proteins by TEV, many of these AIPs will bind to the receptors quite quickly (eg. 8 seconds). Our volume is determined by the distance that AIPs could travel outwards by diffusion within that short time. In this way, we are sure that the concentration of AIPs outside our control volume after a given time is approximately 0.
This approach is not very accurate and can lead us to false negative conclusions (as in reality there will be a concentration gradient, with highest conentration on the cell wall).
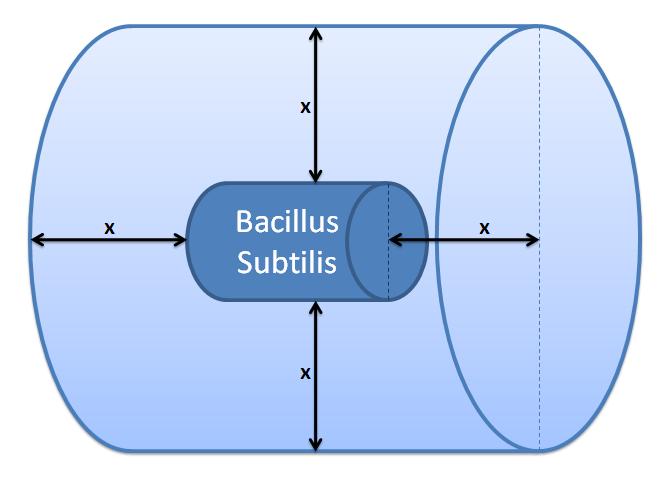
|
Control volume (Volume of B.sub to be excluded.
x indicates the distance travelled by AIPs from
the surface in time t).
|
Difussion distance was calculated using Fick's 1st Law: x=√2Dt, where: x - diffusion distance, D - diffusion constant, t - time of diffusion
Daverage = 10.7×10-11 m2s-1 for a protein in agarose gel for pH=5.6 [3]
t = 8s (arbitrarily chosen)
This gives: x = 4.14×10-5m
The control volume can be calculated by adding 2x to the length and the diamter of the cell.
This gives a control volume (CV) = 4.81×10-7m3
We realized that our initial choice of control volume was not accurate because we had assumed that the bacteria was the medium. However, in reality bacteria live in colonies very close to each other. Since our bacteria was meant to be used in suspension we had to reconsider this issue.
Using CFU to estimate the spacing between cells
CFU stands for Colony-forming unit. It is a measure of bacterial numbers. For liquids, CFU is measured per ml.
We already have data of CFU/ml from the Imperial iGEM 2008 team, so we could use this data to estimate the number of cells in a given volume using a spectrometer at 600nm wavelength.
The graph below is taken from the Imperial iGEM 2008 Wiki page [4].
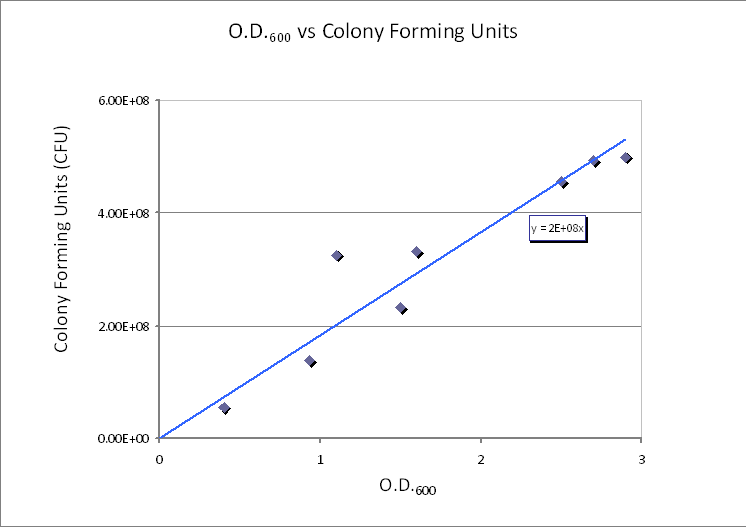
|
| CFU/ml vs. Optical Density at 600nm (OD600).
|
The graph shows values of CFU/ml for different optical densities. The range of CFU/ml is therefore between 0.5×108 - 5×108.
In this calculation, we assumed that only one cell will grow and become one colony (i.e. no more than one cell will form no more than one colony). Therefore, the maximum number of cells in 1ml of solution is 5×108. Taking the volume of 1 ml = 10-3 dm3 and dividing by the (maximum) number of cells in 1ml gives the average control volume (CV) around each cell: 2×10-12 dm3/cell. For simplicity, we choose the control volume to be cubic. Taking the third root of this value gives the length of each side of the control volume.
Side length of CV = y = 1.26×10-4 dm = 1.26×10-5 m.
Choice of Control Volume allows simplifications
- Firstly, assume that the cells will be placed in the centre of the CV. Hence, after cleavage the protein will have an average distance of y/2 to travel in order to cross the boundary of the CV. This is calculated to happen within 0.18s. Even if the bacterium was not placed in the centre of the CV, the protein will travel from one end of the cube to the other in less than one second (~0.74s). Hence, it will take between 0.18 and 0.74s for the concentration of AIPs around the cell to be uniform. Noticing that these time values are very small, we can approximate our model to have a uniform concentration across the volume. Since we are underestimating the value of AIP concentration right next to the cell's surface, we are overestimating the time required for the AIP concentration to reach the threshold level.
- We can neglect the diffusive fluxes across the CV border (see figure below). Assuming that adjacent cells are producing the peptide at the same rate and that the concentration of TEV is the same around the cell, then the fluxes should be of the same value giving a net flux of zero. Hence, we can neglect diffusion and have our model limited to one bacterium.
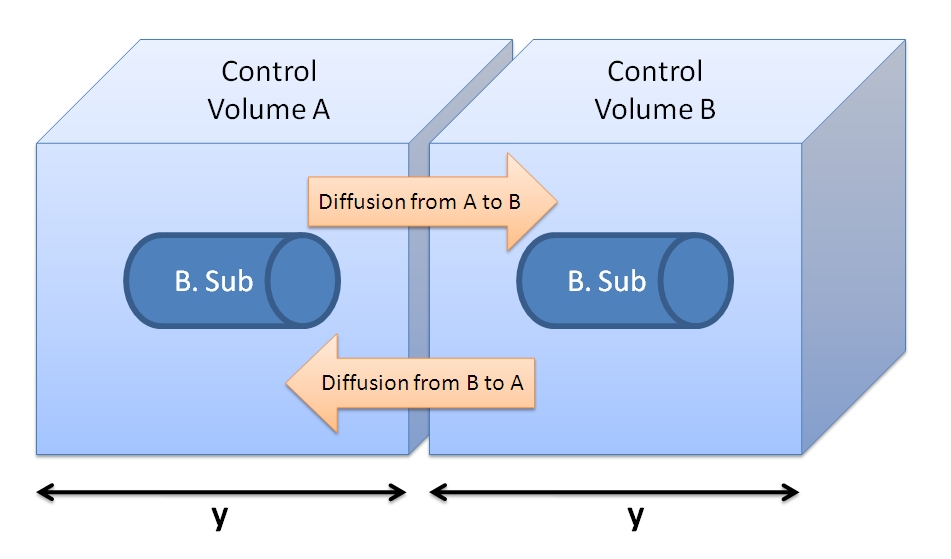
|
| Figure showing two cells with their control volumes.
|
Limitations resulting from our assumptions
Most of our assumptions concerning the control volume were plausible due to the careful choice of cell density = 5×108 CFU/ml.
If the density is varied significantly, then our simplifications might not hold any longer. However, this does not mean that our system cannot function for lower cell densities. Our model might not be very accurate for predicting situations with cell densities that are much higher or lower than 5×108 CFU/ml.
It was decided that the model should not be used for cell densities lower 107 CFU/ml. Below this value AIPs take more than 1 second to diffuse accross half of a side-length of the control volume (assuming that the cell is inside control volume). We agreed that below 10^7 CFU/ml the approximation about uniform concentration throughout the control volume could be wrong and that the concentration gradients could become more significant. If our model was applied to this particular situation, it would possibly overestimate the time taken to activate the receptor.
It is not possible to increase the cell density by more than 109 CFU/ml because of the size of a cell.
5. Protein production
- The paper mentions that each cell displays 2.4×105 peptides. [2]
- 2.4×105 molecules = 2.4×105/6.02×1023 mol = 0.398671×10-18 mol
- Volume of B.sub: 2.79×10-15 dm3
- Concentration = [mol/L]
- c = 1.4289×10-4 mol/dm3. This is the concentration of protein that will be displayed on a single cell of B.sub.
Hence, we can deduce that the final concentration that the protein expression will tend to is: c = 1.4289×10-4 mol/dm3 = cfinal.
Therefore, we can model the protein production by transcription and translation and adjust the production constants so that the concentration will tend towards cfinal.
Using a similar model to the simple production of Dioxygenase for the Output Amplification Model (Model preA), we obtain the following graph:
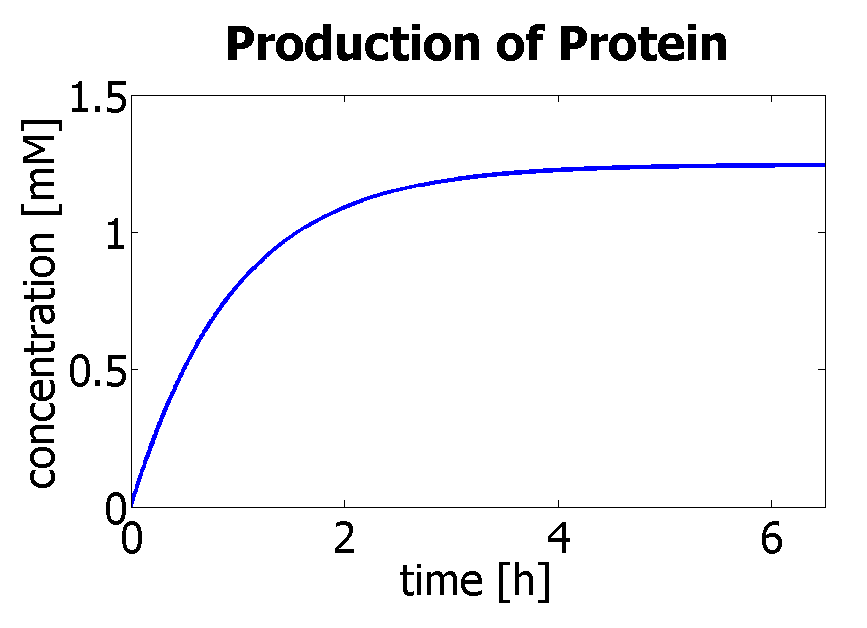
|
| Production of protein by transcription and translation.
|
The degradation rate was kept constant, and the production rate was changed according to the final concentration.
Protein production in Control Volume
The previously determined constants of protein production in B.sub to obtain the concentration of proteins are not valid in the Control Volume. It has to be adjusted (multiplied) by the following factor:
factor=Vbacillus/VCV = 1.4×10-3 (for the particular numbers presented above)
|

|
| Production of protein by transcription and translation in control volume.
|
References
- Havarstein, L., Coomaraswamy, G. & Morrison, D. (1995) An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. [Online] 92, 11140-11144. Available from: http://ukpmc.ac.uk/backend/ptpmcrender.cgi?accid=PMC40587&blobtype=pdf [Accessed 27th August 2010]
- Kobayashi, G. et al (2000) Accumulation of an artificial cell wall-binding lipase by Bacillus subtilis wprA and/or sigD mutants. FEMS Microbiology Letters. [Online] 188(2000), 165-169. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2000.tb09188.x/pdf [Accessed 27th August 2010]
- Gutenwik, J., Nilsson, B. & Axelsson, A. (2003) Determination of protein diffusion coefficients in agarose gel with a diffusion cell. Biochemical Engineering Journal. [Online] 19(2004), 1-7. Available from: http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6V5N-4B3MXDC-2-K&_cdi=5791&_user=217827&_pii=S1369703X03002377&_origin=search&_coverDate=07%2F01%2F2004&_sk=999809998&view=c&wchp=dGLzVtb-zSkzS&md5=c17d0e7320f03931006f9b1a10a438b9&ie=/sdarticle.pdf [Accessed August 20th 2010]
- Imperial College London (2008) Biofabricator Subtilis - Designer Genes. [Online] Available from: https://2008.igem.org/Imperial_College/18_September_2008 [Accessed 1st September 2010]
|
 "
"








