Team:INSA-Lyon/Project/Stage3/Results
From 2010.igem.org
Results
Curli characterization
We studied the Curli promoter thanks to the PHL1273 chassis, a mutant OmpR234 bacteria, which contains a reporter gene under the Curli promoter control. This OmpR234 mutation is required for the high expression of the Curli promoter. A modified GFP is used as a reporter gene. This unusual GFP is unstable and has a half-life of 40 min. The fluorescence of the culture is proportionnal to the quantity of GFP and thus, to the activity of the Curli promoter. We chose to study the influence of shaking speed, temperature and osmotic pressure on the activity of this promoter. For all the experiments, we used the same protocol, (the GFP was quantified by fluorescence spectrophotometry, see Protocols). We were three operators to do the same experiment at the same time.
The negative control was a PHL818 chassis, without the reporter plasmid, but which also contains the OmpR234 mutation. The plasmid with Curli promoter and GFP gene was introduced in this chassis in order to create PHL1273 strain.
Influence of the shaking speed
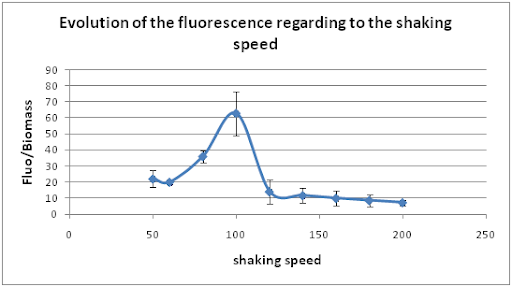
First of all, cultures were in a waterbath at 28°C and only the shaking speed was changed. We have tried 9 different shaking speeds from 50 to 200 rpm.
The curve shows that our system presents the nice property to have an optimal shaking speed at 100 rpm when the temperature is 28°C.
Influence of the temperature
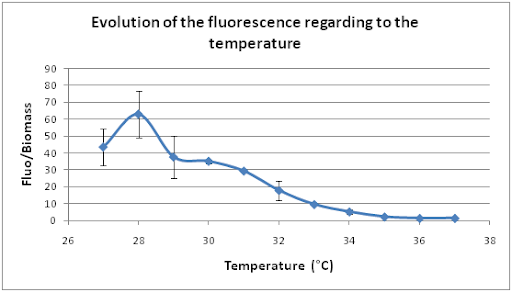
The next series of cultures were in a waterbath with a shaking speed of 100 rpm and we modified the temperature from 27°C to 37°C, degree by degree.
The optimal temperature seems to be 28°C. So our system presents also an optimal response according to the temperature.
For both series of measurements, GFP fluorescence was very low, while the shaking speed and the temperature were high. Notably, when the temperature is higher than 36°C, the GFP fluorescence is the same as the negative control.
Influence of the osmotic pressure
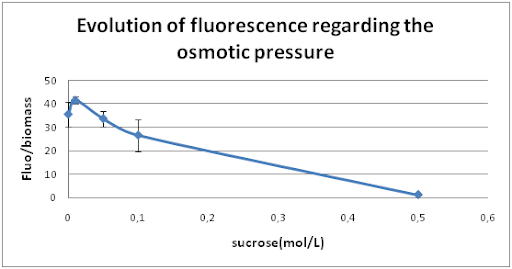
Then, we studied the influence of osmotic pressure on our promoter. We modified the osmotic pressure by changing the sucrose concentration of the culture media. We chose 5 different concentrations in sucrose : 0M - 0,01M - 0,05M - 0,1M – 0,5M. The samples were incubated at 28°C with a shaking speed of 100rpm (previous optimal conditions).
We deduced from this curve that when the osmotic pressure is decreased, the promoter Curli is less active.
In conclusion, the Curli promoter is very responsive at different conditions of culture: shaking speed, temperature and osmotic pressure. Indeed, a gene under the Curli promoter control can be switched ON or OFF by acting on temperature or speed shaking or osmotic pressure. This system is so very versatile and can respond to different environmental signals.
OmpR234 characterization
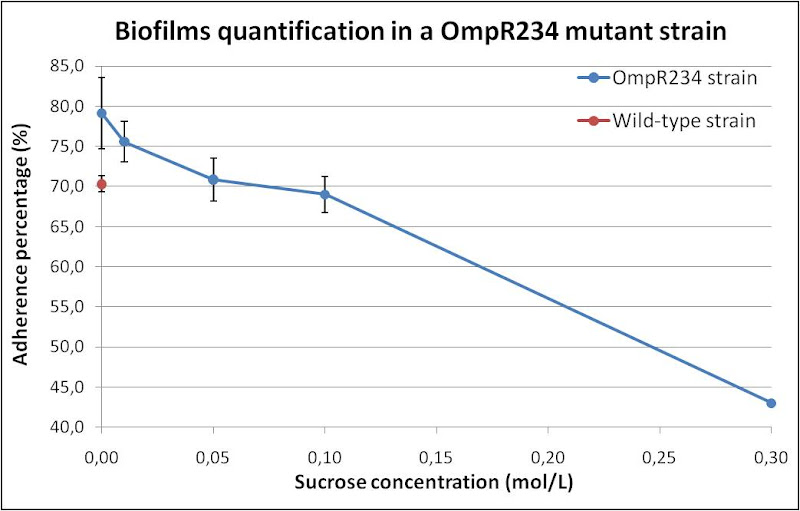
We managed a quantification of biofilms formation of a mutant OmpR234 strain (PHL818 chassis, more explained above), versus a wild-type strain (MG1655), at 30°C, in a 24-well hydrophobic polystyrene maden plate. Optimal condition to observe biofilms is a growth in minimal medium (M63/2), supplemented with a carbon source (glucose 0,2g/L). We modified osmotic pressure as the same way as performed with PHL1273 (sucrose concentration, see above). The purpose of this experiment is to make in evidence the osmolarity influence on the capacity to OmpR234 to induce Curli adherent structures.
As we can see, a decreasing adherence percentage of OmpR234 mutant is observed (about 40%), while the osmotic pressure is decreased (increasing medium osmolarity). The most important biofilm formation is seen without any osmolyte (sucrose 0mol/L). We tested the wild-type strain for adherence without sucrose. We observed that the OmpR234 mutation induced a slight increase in biofilm formation (about 7%). This experiment is a preliminary characterisation of our OmpR234 BioBrick, as the part was designed from this genomic mutant protein (see Design of OmpR234).
Thus, we expected to have a higher effect with a high-copy plasmid containing our OmpR234 BioBrick (under a constitutive promoter for example).
 "
"


