Team:INSA-Lyon/Project/Stage1/Strategy
From 2010.igem.org
| Line 49: | Line 49: | ||
<div style="text-align:center;"> | <div style="text-align:center;"> | ||
| - | <img class= "pili" src="https://static.igem.org/mediawiki/2010/0/0f/Pili.png" alt="Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares)" Title="Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares)"/> <br/> | + | <img class= "pili" src="https://static.igem.org/mediawiki/2010/0/0f/Pili.png" alt="Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares)" Title="Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares)" /> <br/> |
| - | + | </a> | |
<p style="text-align:center; font-size:0.9em;"><em>Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares) </em></p> | <p style="text-align:center; font-size:0.9em;"><em>Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares) </em></p> | ||
| - | <br/><br/> | + | <br/><br/></div> |
<p>We send this sequence to GeneCust to be synthesized. The product of this synthesis had been transformed in competent bacteria NM522.</p> | <p>We send this sequence to GeneCust to be synthesized. The product of this synthesis had been transformed in competent bacteria NM522.</p> | ||
<p>However this plasmid doesn’t match with the iGEM criteria. Indeed some forbidden restriction sites were present in the sequence.</p><br/> | <p>However this plasmid doesn’t match with the iGEM criteria. Indeed some forbidden restriction sites were present in the sequence.</p><br/> | ||
| Line 62: | Line 62: | ||
<p> | <p> | ||
<h4>Design and PCR</h4><br> | <h4>Design and PCR</h4><br> | ||
| - | <p>We designed a phaC gene in standard BioBrick from the pILI1 sequence: as there were | + | <p> |
| - | two Pst1 and one Not1 sites inside the gene, we looked for silent mutations in those sites and | + | We designed a <i>phaC</i> gene in standard BioBrick from the pILI1 sequence: as there were two Pst1 and one Not1 sites inside the gene, we looked for silent mutations in those sites and |
modified the sequence to make the sites disappear. Then we added the sequence of iGEM prefix at the beginning and the suffix one at the end in order to have the sequence in the standard BioBrick.</p> | modified the sequence to make the sites disappear. Then we added the sequence of iGEM prefix at the beginning and the suffix one at the end in order to have the sequence in the standard BioBrick.</p> | ||
<br/> | <br/> | ||
<div style="text-align:center;"> | <div style="text-align:center;"> | ||
| - | <img src="http://lh3.ggpht.com/_Uc3bmii-yi0/TMgfmJf-2aI/AAAAAAAAAmY/MmQgbaghPRs/sequenceStrategieStage1.PNG" alt="Sequence" title="Sequence" > | + | <img src="http://lh3.ggpht.com/_Uc3bmii-yi0/TMgfmJf-2aI/AAAAAAAAAmY/MmQgbaghPRs/sequenceStrategieStage1.PNG" alt="Sequence" title="Sequence" /> |
| - | <br/> | + | </div><br/> |
| - | + | ||
<br/><br/> | <br/><br/> | ||
| - | <p>In parallel, we performed a PCR to get < | + | <p>(The A from the ATG of the coding sequence completes the Xba1 site)</p> |
| - | software to make them the most stable and the most specific that we could, adding the prefix | + | <p>In parallel, we performed a PCR to get <i>phaA</i> and <i>phaB</i> from the pILI1 sequence because no iGEM restriction sites were on the sequence and the sequences of those genes were short enough. We designed them using a software to make them the most stable and the most specific that we could, adding the prefix sequence at the beginning of the forward primer and the suffix sequence at the end of the reverse primer. Then we wanted to ligate the three genes in the original order.</p> |
| - | sequence at the beginning of the forward primer and the suffix sequence at the end of the reverse | + | |
| - | primer. | + | |
| - | Then we wanted to ligate the three genes in the original order.</p> | + | |
<div style="text-align:center;"> | <div style="text-align:center;"> | ||
| - | <img src="http://lh6.ggpht.com/_zZap34AU7o8/TMc9ZuoUPMI/AAAAAAAAABA/agtSWcFEkuc/s640/operon_seq_wiki.png" alt="Strategy with PCR" title="First strategy" > <br/> | + | <img src="http://lh6.ggpht.com/_zZap34AU7o8/TMc9ZuoUPMI/AAAAAAAAABA/agtSWcFEkuc/s640/operon_seq_wiki.png" alt="Strategy with PCR" title="First strategy" /> <br/> |
<p style="text-align:center; font-size:0.9em;"><em>First strategy for ligation </em></p> | <p style="text-align:center; font-size:0.9em;"><em>First strategy for ligation </em></p> | ||
| - | |||
</div> | </div> | ||
<br> | <br> | ||
| Line 90: | Line 85: | ||
<p>Unfortunately, we never managed to cut the phaC gene we found by the Xba1 enzyme so we couldn’t use our new | <p>Unfortunately, we never managed to cut the phaC gene we found by the Xba1 enzyme so we couldn’t use our new | ||
operon.</p> | operon.</p> | ||
| - | <p>Our two final strategies were to ligate phaC with either PCR product either iGEM < | + | <p>Our two final strategies were to ligate phaC with either PCR product either iGEM <i>phaA</i> and <i>phaB</i>. |
With more time we could have designed a primer with the right restriction sites but we were not | With more time we could have designed a primer with the right restriction sites but we were not | ||
| - | sure the < | + | sure the <i>phaC</i> gene had just this problem.</p> |
| - | <br/> | + | <br /> |
| - | <br> | + | <br /> |
</div> | </div> | ||
| - | |||
<p style="text-align:center"><a href="#top">Top of Page</a></p> | <p style="text-align:center"><a href="#top">Top of Page</a></p> | ||
| + | <div id="corps4" style="border:0px;"> | ||
| - | |||
<div style="float:justify;border:solid 0px green;> <!-- mettre 1px si cadre --> | <div style="float:justify;border:solid 0px green;> <!-- mettre 1px si cadre --> | ||
-moz-border-radius: 15px; | -moz-border-radius: 15px; | ||
Revision as of 15:50, 27 October 2010
Strategy
Complete plasmid synthesis : pILI1
In 1988, Slater et al. (Journal of Bacteriology) developed the plasmid pSB20. Then the construction of a total DNA bank of Alcaligenes eutrophus permits to identified two restriction sites which surround the three genes and their promoter: KpnI and EcoRI. With the help of a software (Serial Cloner) and the sequence of Ralstonia eutropha (old named Alcaligenes eutrophus), a 5800 pb length sequence had been isolated. It contains the promoter and the phaCAB genes. This sequence had been inserted into a plasmid pUC18. A HindIII site had been added before the promoter and also a unique restriction site XhoI between the promoter and the operon (so we could change the promoter). This is our plasmid pILI1.

Plasmid pILI1 map (Adapted from PlasMapper and BPROM online softwares)
We send this sequence to GeneCust to be synthesized. The product of this synthesis had been transformed in competent bacteria NM522.
However this plasmid doesn’t match with the iGEM criteria. Indeed some forbidden restriction sites were present in the sequence.
PhaCAB Operon: two strategies to construct a standard BioBrick
Design and PCR
We designed a phaC gene in standard BioBrick from the pILI1 sequence: as there were two Pst1 and one Not1 sites inside the gene, we looked for silent mutations in those sites and modified the sequence to make the sites disappear. Then we added the sequence of iGEM prefix at the beginning and the suffix one at the end in order to have the sequence in the standard BioBrick.
(The A from the ATG of the coding sequence completes the Xba1 site)
In parallel, we performed a PCR to get phaA and phaB from the pILI1 sequence because no iGEM restriction sites were on the sequence and the sequences of those genes were short enough. We designed them using a software to make them the most stable and the most specific that we could, adding the prefix sequence at the beginning of the forward primer and the suffix sequence at the end of the reverse primer. Then we wanted to ligate the three genes in the original order.
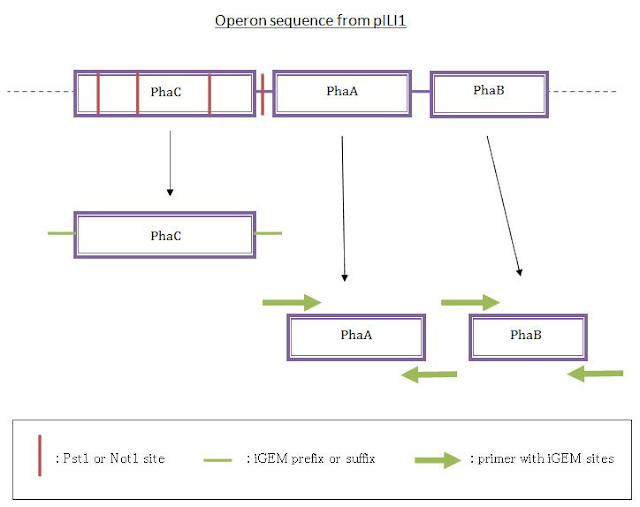
First strategy for ligation
Using iGEM Regestry
We also got the three genes directly from the iGEM registry, and we tried to ligate them. The BioBricks we used are : BBa_K156012 for phaA, BBa_K156013 for phaB and BBa_K156014 for phaC.
Unfortunately, we never managed to cut the phaC gene we found by the Xba1 enzyme so we couldn’t use our new operon.
Our two final strategies were to ligate phaC with either PCR product either iGEM phaA and phaB. With more time we could have designed a primer with the right restriction sites but we were not sure the phaC gene had just this problem.
 "
"


