Team:HokkaidoU Japan/Notebook/August18
From 2010.igem.org
(Difference between revisions)
(→Preparation of DNA Solution for Ligation) |
(→Ligation & Transformation) |
||
| Line 77: | Line 77: | ||
|} | |} | ||
| - | ===Ligation | + | ===Ligation and Transformation=== |
{|style="text-align:center;" class="protocol" | {|style="text-align:center;" class="protocol" | ||
!Reagent | !Reagent | ||
| Line 95: | Line 95: | ||
|} | |} | ||
| - | * | + | * Incubated at 16C for 30 min |
| - | * competent cell | + | * Transformation: Added all to 50 uL of competent cell |
| - | * | + | * Incubated at 0C for 30 min |
| - | * | + | * Heat shocked at 42C for 60 sec |
* 5 min on ice | * 5 min on ice | ||
| - | * | + | * Added 100 uL of LB |
| - | * | + | * Incubated at 37C for 120 min |
| - | * | + | * Spread onto the LBC plate |
| - | * | + | * ncubated at 37C for 15~20 hrs |
=RBS 再リベンジ= | =RBS 再リベンジ= | ||
Revision as of 16:33, 21 September 2010
RBS digestion: Revenge
| Reagent | Amount |
|---|---|
| 1-2M | 10 uL |
| DW | 4 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
→Incubated at 37C for 60 min
- Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed
→Extracted for gel
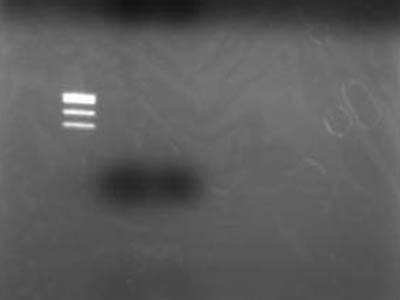
→Electrophoresed 10 uL of Extracted DNA
- Used TSUDA Marker 1
Failure
- After better inspection of Kit specs it came to lite that retreavel rate for 50 bp and less is 26%
- RBS is just quite small when cut
Ligation
Preparation of DNA Solution for Ligation
| Part | By comparison toLambda/Hind III (ng/10 uL) | ng/uL | ratio | size (bp) | (ng) required | (uL) used |
|---|---|---|---|---|---|---|
| Vector | 50 ng/10 uL | 5 ng/uL | 1 | 2996 bp | 10 ng | 2 uL |
| RFP | 250 ng/10 uL | 25 ng/uL | 2 | 700 bp | 7 ng | 0.3 uL |
| double terminator | 5 ng/10 uL | 0.5 ng/uL | 2 | 200 bp | 2 ng | 4 uL |
| Total | 6.3 uL | |||||
Ligation and Transformation
| Reagent | Amount |
|---|---|
| DNA solution | 6.3 uL |
| Ligation solution | 6.3 uL |
| T4 ligase | 1 uL |
| Total | 13.6 uL |
- Incubated at 16C for 30 min
- Transformation: Added all to 50 uL of competent cell
- Incubated at 0C for 30 min
- Heat shocked at 42C for 60 sec
- 5 min on ice
- Added 100 uL of LB
- Incubated at 37C for 120 min
- Spread onto the LBC plate
- ncubated at 37C for 15~20 hrs
RBS 再リベンジ
Digestion
| Reagent | Amount |
|---|---|
| (RBS)1-2M | 10 uL |
| DW | 4 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
→37℃,60 min
エタ沈
- 2 uLの3 M 酢酸ナトリウムを加えた
- 44 uLのエタノールを加えた
- 液体窒素の中で凍らせた(1.5 mLのチューブに移す)
- 溶かしてから15,996 rpm @4℃で5分間遠心した
- 上清をほかのチューブに移した(一応これも遠心し,上清を捨て,同じ操作をした)
- 100 uLの70%エタノールで壁をリンスし,15,996 rpm @4℃で5分間遠心した
- 上清を捨て,真空デシケータで乾燥させた
- TE 5 uLで溶かした
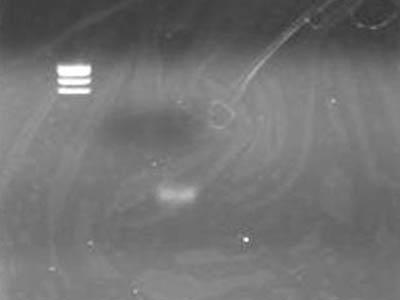
電気泳動
- TEで溶かしたDNA Solution 1 uLに6x SB 1 uLを加えた
- 最初にとった上清も同じ操作をした
| Lane | DNA |
| 2 | TSUDA Marker 1 |
| 3 | 上清 |
| 4 | DNA solution |
- DNA solutionにしっかり回収されていた
 "
"