Team:HokkaidoU Japan/Notebook/August18
From 2010.igem.org
(Difference between revisions)
(→Ethanol Precipication) |
|||
| (3 intermediate revisions not shown) | |||
| Line 27: | Line 27: | ||
|style="border-top:1px solid #996"|'''20 uL''' | |style="border-top:1px solid #996"|'''20 uL''' | ||
|} | |} | ||
| - | + | ->Incubated at 37C for 60 min | |
* Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed | * Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed | ||
| - | + | ->Extracted for gel <br> | |
| - | + | ->Electrophoresed 10 uL of Extracted DNA | |
* Used [https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png TSUDA Marker 1] | * Used [https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png TSUDA Marker 1] | ||
| Line 132: | Line 132: | ||
|style="border-top:1px solid #996"|'''20 uL''' | |style="border-top:1px solid #996"|'''20 uL''' | ||
|} | |} | ||
| - | + | ->Incubated at 37C for 60 min | |
==[[Team:HokkaidoU_Japan/Protocols|Ethanol Precipication]]== | ==[[Team:HokkaidoU_Japan/Protocols|Ethanol Precipication]]== | ||
| - | * Added 2 uL of sodium acetate( | + | * Added 2 uL of sodium acetate(3 M) |
* Added 44 uL of Ethanol | * Added 44 uL of Ethanol | ||
* Transfered to 1.5 mL tube and frozen with liquid nitrogen | * Transfered to 1.5 mL tube and frozen with liquid nitrogen | ||
| - | * Melted and centrifuged at | + | * Melted and centrifuged at 15000 rpm, 4C for 5 min |
* Transfered supernatant to another tube | * Transfered supernatant to another tube | ||
** Driven by precaution centrifuged the supernatant and discarded it's supernatant | ** Driven by precaution centrifuged the supernatant and discarded it's supernatant | ||
| - | * Rinsed the tube walls with 100 uL of 70% Ethanol and centrifuged at | + | * Rinsed the tube walls with 100 uL of 70% Ethanol and centrifuged at 15000 rpm, 4C for 5 min |
* Discarded the supertenant and dried via vacuum desiccator | * Discarded the supertenant and dried via vacuum desiccator | ||
* Melted in 5 uL of TE | * Melted in 5 uL of TE | ||
Latest revision as of 13:28, 27 October 2010
RBS digestion: Revenge
| Reagent | Amount |
|---|---|
| 1-2M | 10 uL |
| DW | 4 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
->Incubated at 37C for 60 min
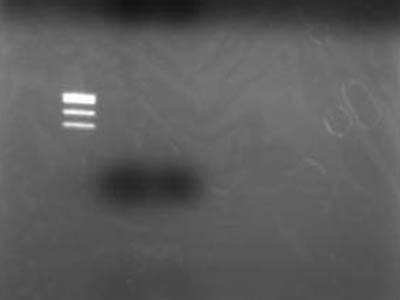
- Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed
->Extracted for gel
->Electrophoresed 10 uL of Extracted DNA
- Used TSUDA Marker 1
Failure
- After better inspection of Kit specs it came to lite that retreavel rate for 50 bp and less is 26%
- RBS is just quite small when cut
Ligation
Preparation of DNA Solution for Ligation
| Part | By comparison toλ/Hind III (ng/10 uL) | ng/uL | ratio | size(bp) | required(ng) | used(uL) |
|---|---|---|---|---|---|---|
| Vector | 50 ng/10 uL | 5 ng/uL | 1 | 2996 bp | 10 ng | 2 uL |
| RFP | 250 ng/10 uL | 25 ng/uL | 2 | 700 bp | 7 ng | 0.3 uL |
| terminator | 5 ng/10 uL | 0.5 ng/uL | 2 | 200 bp | 2 ng | 4 uL |
| Total | 6.3 uL | |||||
Ligation and Transformation
| Reagent | Amount |
|---|---|
| DNA solution | 6.3 uL |
| Ligation solution | 6.3 uL |
| T4 ligase | 1 uL |
| Total | 13.6 uL |
- Incubated at 16C for 30 min
- Transformation: Added all to 50 uL of competent cell
- Incubated at 0C for 30 min
- Heat shocked at 42C for 60 sec
- 5 min on ice
- Added 100 uL of LB
- Incubated at 37C for 120 min
- Spread onto the LBC plate
- Incubated at 37C for 20 hrs
RBS Retry
Digestion
| Reagent | Amount |
|---|---|
| (RBS)1-2M | 10 uL |
| DW | 4 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
->Incubated at 37C for 60 min
Ethanol Precipication
- Added 2 uL of sodium acetate(3 M)
- Added 44 uL of Ethanol
- Transfered to 1.5 mL tube and frozen with liquid nitrogen
- Melted and centrifuged at 15000 rpm, 4C for 5 min
- Transfered supernatant to another tube
- Driven by precaution centrifuged the supernatant and discarded it's supernatant
- Rinsed the tube walls with 100 uL of 70% Ethanol and centrifuged at 15000 rpm, 4C for 5 min
- Discarded the supertenant and dried via vacuum desiccator
- Melted in 5 uL of TE
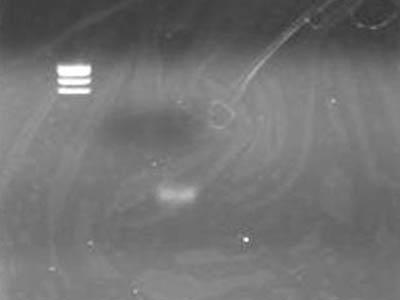
Electrophoresis
- Added 1 uL of 6x SB to DNA Solution of 1 uL
- Did the same to supernatant retreaved for precaution
| Lane | DNA |
| 2 | TSUDA Marker 1 |
| 3 | supernatant |
| 4 | DNA solution |
- DNA solution band is visible
- Looks OK
 "
"