Team:HokkaidoU Japan/Notebook/August16
From 2010.igem.org
Restriction Enzyme Activity Check
Restriction Enzyme Digestion
EcoR I, Xba I, Pst I
| Reagent | Amount |
|---|---|
| pUC119 | 1 uL |
| DW | 7 uL |
| 10x M Buffer | 1 uL |
| Restriction Enzyme | 1 uL |
| Total | 10 uL |
- Made solution for each EcoR I, Xba I, Pst I
- incubated at 37C for 60 min
Spe I
| Reagent | Amount |
|---|---|
| 1-1A | 1 uL |
| DW | 7 uL |
| 10x M Buffer | 1 uL |
| Restriction Enzyme | 1 uL |
| Total | 10 uL |
- pUC119 doesn't have Spe I restriction sites, so we used mini preped plasmid with biobrick insert
- incubated at 37C for 60 min
Control
- Added 1 uL of 6x Sample Buffer to 1 uL of pUC119
- Also added 1 uL of 6x Sample Buffer to 1 uL of 1-1A
- Incubated at 37C for 60 min
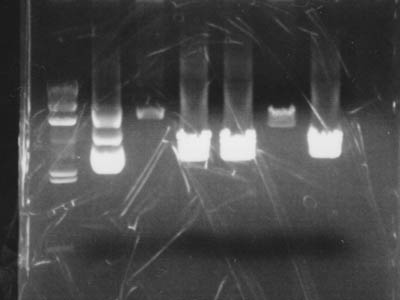
Electrophoresis
Added 2 uL of 6x Sample Buffer to digested solution and performed electrophoresis
| Lane | DNA |
|---|---|
| 1 | Lambda/HindIII |
| 2 | pUC119 control |
| 3 | 1-1A control |
| 4 | pUC119 + EcoR I |
| 5 | pUC119 + Xba I |
| 6 | 1-1A + Spe I |
| 7 | pUC119 + Pst I |
| 8 | Empty |
Restriction Enzyme (EcoR I, Pst I) Digestion of the vector
| Reagent | Amount |
|---|---|
| pSB1C3 | 4 uL |
| DW | 10 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| EcoR I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
Restriction Enzyme (Xba I, Pst I) Digestion of the Parts
RBS
| Reagent | Amount |
|---|---|
| 1-2M | 2.5 uL |
| DW | 11.5 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
Terminator
| Reagent | Amount |
|---|---|
| 1-23L | 1.5 uL |
| DW | 12.5 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
Restriction Enzyme (EcoR I, Spe I) Digestion of the Parts
Heat shock promotor
| Reagent | Amount |
|---|---|
| 3-1E | 5 uL |
| DW | 9.7 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| EcoR I | 1 uL |
| Spe I | 0.3 uL |
| Total | 20 uL |
RFP
| Reagent | Amount |
|---|---|
| 1-18F | 5 uL |
| DW | 9.7 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| EcoR I | 1 uL |
| Spe I | 0.3 uL |
| Total | 20 uL |
- It was obvious after restriction enzyme activity check that Spe I was week, so additional 0.7 uL was added
 "
"