Team:HokkaidoU Japan/Notebook/August12
From 2010.igem.org
(Difference between revisions)
(New page: {{Template:HokkaidoU_Japan}}) |
|||
| (16 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{Template:HokkaidoU_Japan}} | + | {{Template:HokkaidoU_Japan}}<div class="linkbar"><div class="prev">[[Team:HokkaidoU_Japan/Notebook/August11|August 11]]</div>[[Team:HokkaidoU_Japan/Notebook|Notebook]]<div class="next">[[Team:HokkaidoU_Japan/Notebook/August13|August 13]]</div></div> |
| + | |||
| + | =Electrophoresis 1= | ||
| + | |||
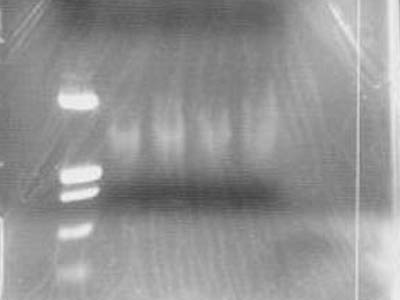
| + | [[Image:HokkaidoU Japan 20100812a.jpg|200px|right|thumb|Electrophoresis of yesterdays ligation of PCR products]] | ||
| + | |||
| + | * Checked the result of yesterdays ligation of PCR products by electrophoresis | ||
| + | * Added 4 uL of 6x Sample Buffer to make 12 uL in total | ||
| + | * Used 20 uL of EtOH | ||
| + | <div style="clear:both"></div> | ||
| + | |||
| + | =[[Team:HokkaidoU_Japan/Protocols|Mini prep]]= | ||
| + | * Used cells incubated for 18h in LB broth | ||
| + | ** [[Team:HokkaidoU_Japan/Parts#BioBricks|1-18F]] didn't grow well | ||
| + | * Centrifuge after adding 430 uL of chloroform、collected supernatant | ||
| + | * Added 2-propanol to [[Team:HokkaidoU_Japan/Parts#BioBricks|1-18F]], but after centrifugation there were no precipitation | ||
| + | ** Melted in 30 uL of TE and did electrophoresis | ||
| + | |||
| + | =Electrophoresis 2= | ||
| + | |||
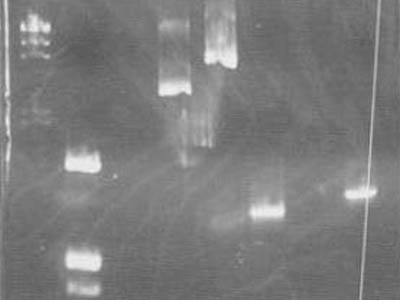
| + | [[Image:HokkaidoU Japan 20100812b.jpg|200px|right|thumb|Take 1]] | ||
| + | [[Image:HokkaidoU Japan 20100812c.jpg|200px|right|thumb|Take 2 after few minutes]] | ||
| + | |||
| + | Checked plasmids gathered via [[Team:HokkaidoU_Japan/Protocols|mini prep]] and [[Team:HokkaidoU_Japan/Protocols|PCR]] products, by electrophoresis. <br> | ||
| + | |||
| + | |||
| + | Because we wont do restriction enzyme digestion reaction system is ass follows. | ||
| + | |||
| + | {| style="text-align:center" class="protocol" | ||
| + | |- | ||
| + | |style="border-bottom:1px solid #996; border-right:1px solid #996;"|'''Lane''' | ||
| + | |style="border-bottom:1px solid #996;"| 1 | ||
| + | |style="border-bottom:1px solid #996;"| 2 | ||
| + | |style="border-bottom:1px solid #996;"| 3 | ||
| + | |style="border-bottom:1px solid #996;"| 4 | ||
| + | |style="border-bottom:1px solid #996;"| 5 | ||
| + | |style="border-bottom:1px solid #996;"| 6 | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"| Parts | ||
| + | | [[Team:HokkaidoU_Japan/Parts#BioBricks|1-18F]] | ||
| + | | [[Team:HokkaidoU_Japan/Parts#BioBricks|2-21H]] | ||
| + | | [[Team:HokkaidoU_Japan/Parts#BioBricks|2-11P]] | ||
| + | | [[Team:HokkaidoU_Japan/Parts#BioBricks|2-24G]] | ||
| + | | [[Team:HokkaidoU_Japan/Parts#BioBricks|1-2M]] | ||
| + | | [[Team:HokkaidoU_Japan/Parts#BioBricks|3-1E]] | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"| DNA solution | ||
| + | | 2 uL | ||
| + | | 2 | ||
| + | | 2 | ||
| + | | 1 | ||
| + | | 1 | ||
| + | | 1 | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"| 6x Sample Buffer | ||
| + | | 0.4 uL | ||
| + | | 0.4 | ||
| + | | 0.4 | ||
| + | | 0.4 | ||
| + | | 0.4 | ||
| + | | 0.4 | ||
| + | |} | ||
| + | * Used markers are [https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png λ/''Hin''dIII] and [https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png pUC119/''Hin''fI] | ||
| + | * Marker in lane 1 ([https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png λ/''Hin''dIII]) leaked out | ||
| + | * Mini prep sample,1-18F band in lane 3 wasn't visible so we [[Team:HokkaidoU_Japan/Protocols|decided]] PCR it tomorrow | ||
| + | * In lane 4 there were some visible plasmid dimers and trimer s | ||
Latest revision as of 07:25, 27 October 2010
Electrophoresis 1
- Checked the result of yesterdays ligation of PCR products by electrophoresis
- Added 4 uL of 6x Sample Buffer to make 12 uL in total
- Used 20 uL of EtOH
Mini prep
- Used cells incubated for 18h in LB broth
- 1-18F didn't grow well
- Centrifuge after adding 430 uL of chloroform、collected supernatant
- Added 2-propanol to 1-18F, but after centrifugation there were no precipitation
- Melted in 30 uL of TE and did electrophoresis
Electrophoresis 2
Checked plasmids gathered via mini prep and PCR products, by electrophoresis.
Because we wont do restriction enzyme digestion reaction system is ass follows.
| Lane | 1 | 2 | 3 | 4 | 5 | 6 |
| Parts | 1-18F | 2-21H | 2-11P | 2-24G | 1-2M | 3-1E |
| DNA solution | 2 uL | 2 | 2 | 1 | 1 | 1 |
| 6x Sample Buffer | 0.4 uL | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
- Used markers are λ/HindIII and pUC119/HinfI
- Marker in lane 1 (λ/HindIII) leaked out
- Mini prep sample,1-18F band in lane 3 wasn't visible so we decided PCR it tomorrow
- In lane 4 there were some visible plasmid dimers and trimer s
 "
"