Team:Heidelberg/Notebook/BSDesign/August
From 2010.igem.org
| (2 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{:Team:Heidelberg/Single_Pagetop|note_BSDesign}} | {{:Team:Heidelberg/Single_Pagetop|note_BSDesign}} | ||
{{:Team:Heidelberg/Side_Top}} | {{:Team:Heidelberg/Side_Top}} | ||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#4e93a4; border:1.53px solid #333333;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#c85000;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July<font color="white">July</font>] | ||
| + | |- style="background:#c85000; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="3"| ||'''1'''||'''2'''||'''3'''||'''4''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 5]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 6]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 7]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 8]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 10]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 11]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 12]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 13]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 14]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 15]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 16]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 17]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 18]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 19]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#20.2F07.2F2010 20]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#21.2F07.2F2010 21]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#22.2F07.2F2010 22]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#23.2F07.2F2010 23]'''||'''24'''||'''25''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#26.2F07.2F2010 26]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#27.2F07.2F2010 27]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#28.2F07.2F2010 28]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#29.2F07.2F2010 29]'''||'''30'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#31.2F07.2F2010 31]'''|| | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | | colspan="7"| | ||
| + | <span style="color:#ffffff">-</span> | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#f09600; border: 1.5px solid #000000;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#f09600;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August<font color="white">August</font>] | ||
| + | |- style="background:#f09600; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="6"| ||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#01.2F08.2F2010 1]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#02.2F08.2F2010 2]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#03.2F08.2F2010 3]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#04.2F08.2F2010 4]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#05.2F08.2F2010 5]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#06.2F08.2F2010 6]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#07.2F08.2F2010 7]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#08.2F08.2F2010 8]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#09.2F08.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#10.2F08.2F2010 10]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#11.2F08.2F2010 11]'''||'''12'''||'''13'''||'''14'''||'''15''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''16'''||'''17'''||'''18'''||'''19'''||'''20'''||'''21'''||'''22''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''23'''||'''24'''||'''25'''||'''26'''||'''27'''||'''28'''||'''29''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''30'''||'''31'''||colspan="5"| | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#f09600; border: 1.5px solid #333333;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#009be1;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September<font color="#ffecba">September</font>] | ||
| + | |- style="background:#009be1; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="2"| ||'''1'''||'''2'''||'''3'''||'''4'''||'''5''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''6'''||'''7'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#08.2F09.2F2010 8]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#09.2F09.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#10.2F09.2F2010 10]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#11.2F09.2F2010 11]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#12.2F09.2F2010 12]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#13.2F09.2F2010 13]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#14.2F09.2F2010 14]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#15.2F09.2F2010 15]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#16.2F09.2F2010 16]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#17.2F09.2F2010 17]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#18.2F09.2F2010 18]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#19.2F09.2F2010 19]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#20.2F09.2F2010 20]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#21.2F09.2F2010 21]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#22.2F09.2F2010 22]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#23.2F09.2F2010 23]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#24.2F09.2F2010 24]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#25.2F09.2F2010 25]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#26.2F09.2F2010 26]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#27.2F09.2F2010 27]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#28.2F09.2F2010 28]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#29.2F09.2F2010 29]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#30.2F30.2F2010 30]'''||colspan="5"| | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="7"| | ||
| + | <span style="color:#ffffff">-</span> | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#f09600; border: 1.5px solid #333333;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#78b41e;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October<font color="white">October</font>] | ||
| + | |- style="background:#78b41e; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="4"| ||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#01.2F10.2F2010 1]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#02.2F10.2F2010 2]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#03.2F10.2F2010 3]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#04.2F10.2F2010 4]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#05.2F10.2F2010 5]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#06.2F10.2F2010 6]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#07.2F10.2F2010 7]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#08.2F10.2F2010 8]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#09.2F10.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#10.2F10.2F2010 10]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#11.2F10.2F2010 11]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#12.2F10.2F2010 12]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#13.2F10.2F2010 13]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#14.2F10.2F2010 14]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#15.2F10.2F2010 15]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#16.2F10.2F2010 16]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#17.2F10.2F2010 17]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#18.2F10.2F2010 18]'''|||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#19.2F10.2F2010 19]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#20.2F10.2F2010 20]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#21.2F10.2F2010 21]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#22.2F10.2F2010 22]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#23.2F10.2F2010 23]'''||'''24''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''25'''||'''26'''||'''27'''||'''28'''||'''29'''||'''30'''||'''31''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="7"| | ||
| + | <span style="color:#ffffff">-</span> | ||
| + | |} | ||
{{:Team:Heidelberg/Side_Bottom}} | {{:Team:Heidelberg/Side_Bottom}} | ||
| Line 199: | Line 281: | ||
<br /> | <br /> | ||
Ligation reaction was incubated at 19 °C for 5 h; 10 ul of each ligation reaction was transformed into E. coli Top10 according to the standard transformation protocol. | Ligation reaction was incubated at 19 °C for 5 h; 10 ul of each ligation reaction was transformed into E. coli Top10 according to the standard transformation protocol. | ||
| - | <br /> | + | <br /><br /><br /> |
| - | <br /> | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <br /> | + | |
---- | ---- | ||
| Line 269: | Line 341: | ||
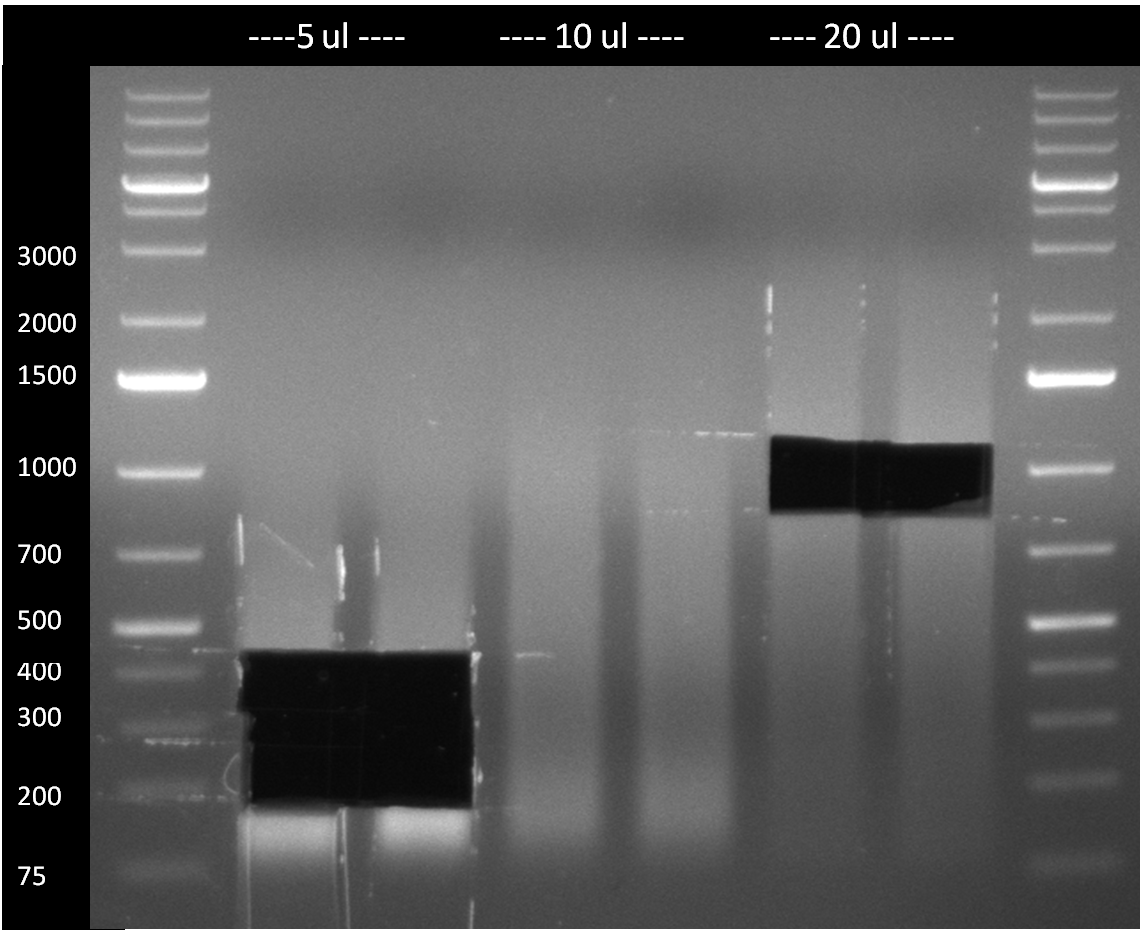
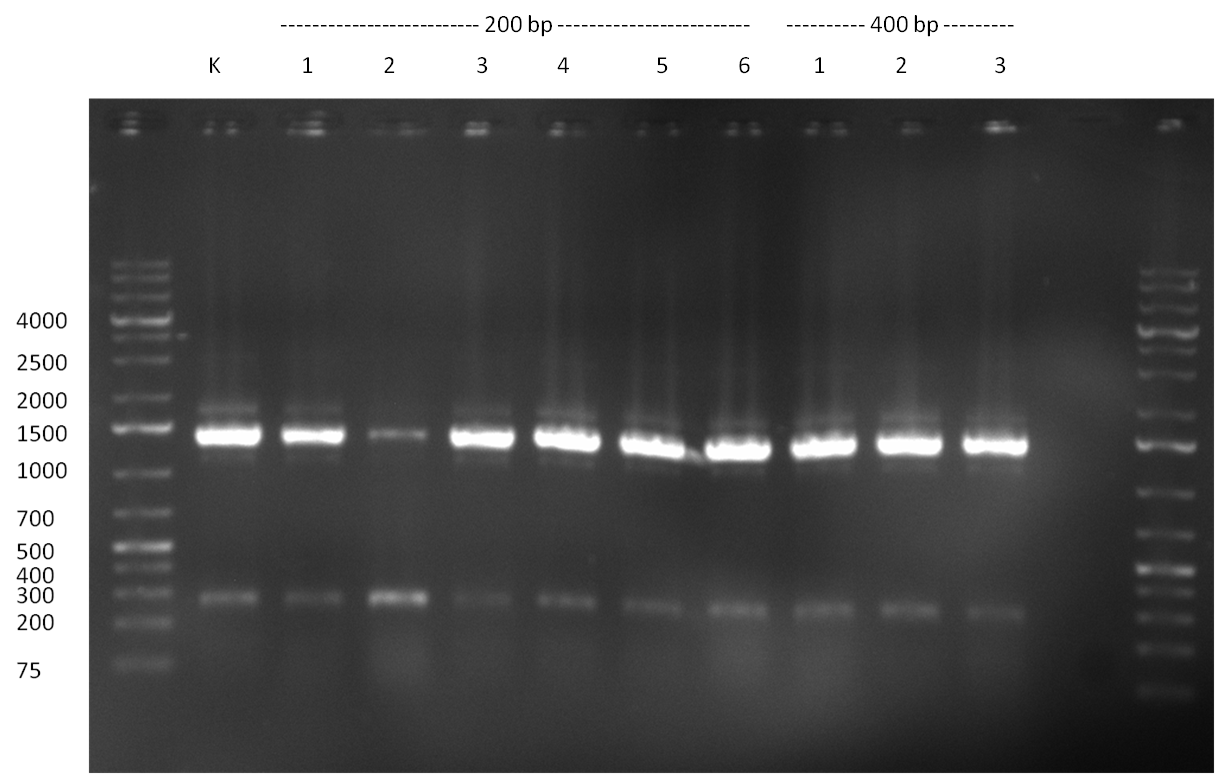
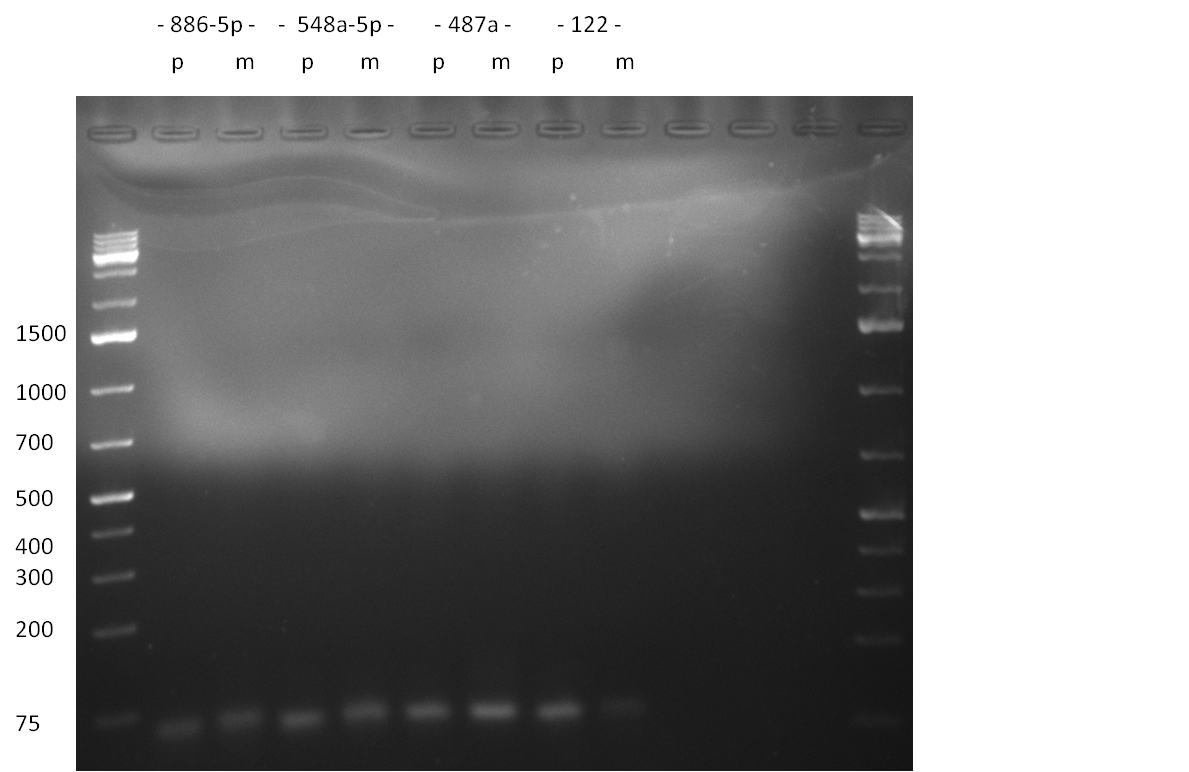
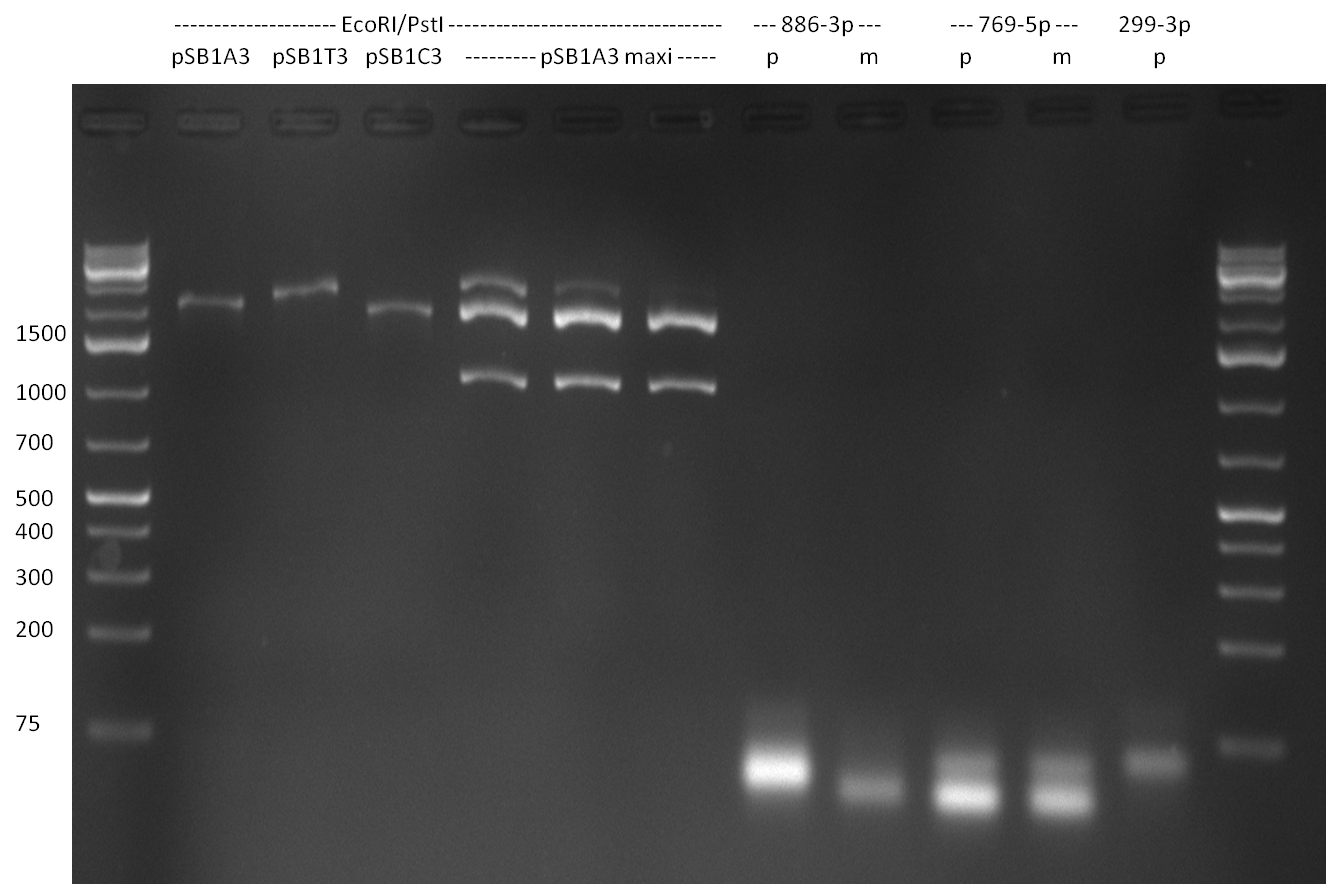
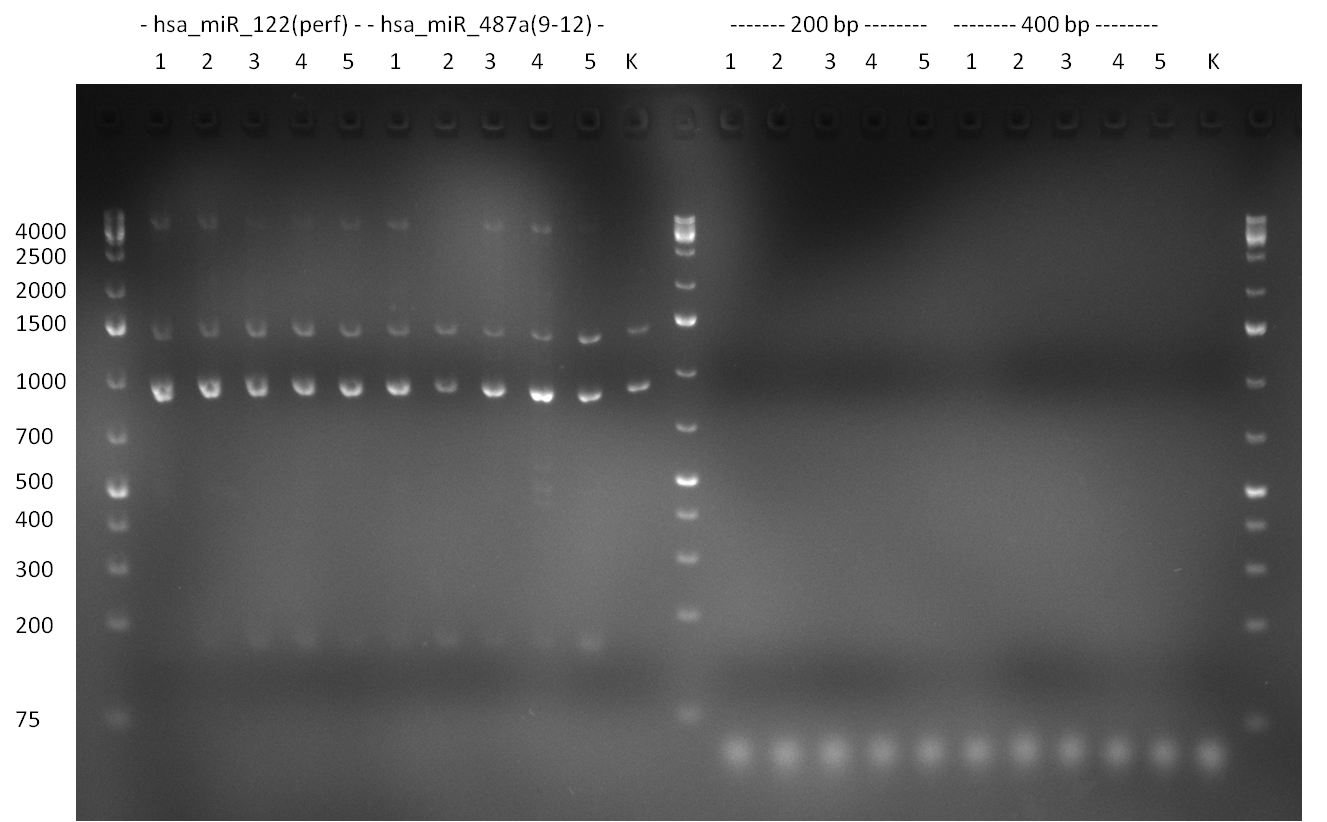
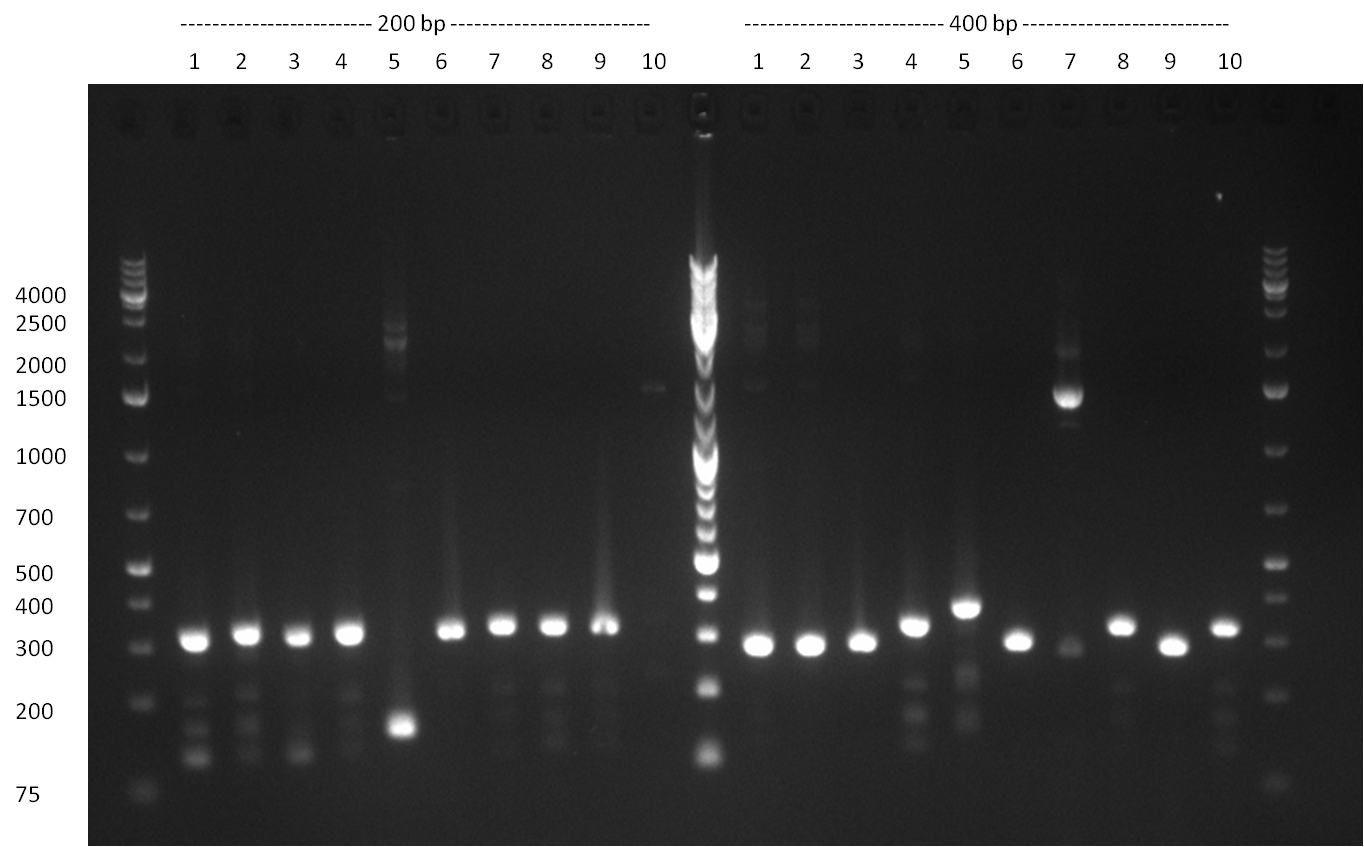
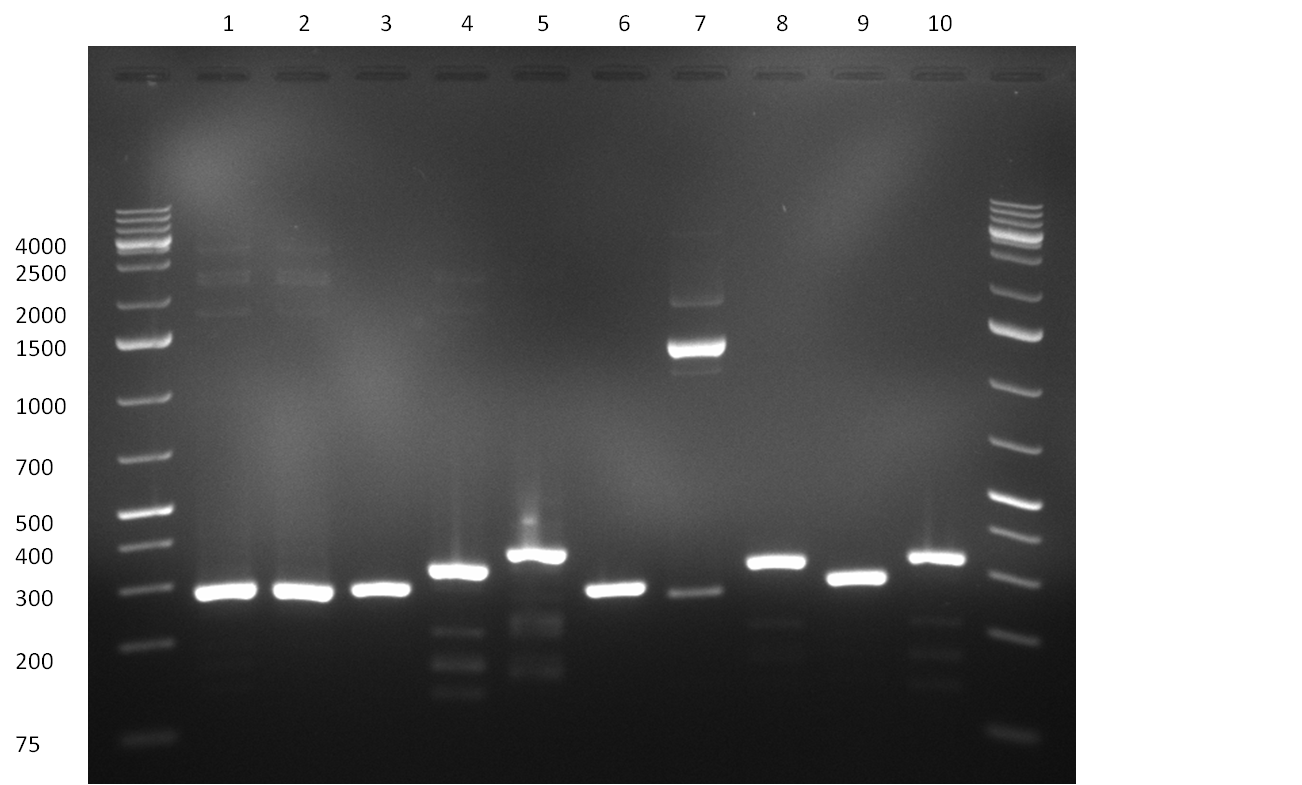
* Picking of 5 additional colonies of the transformation (binding site patterns) from the plates containing the 200 bp and 400 bp constructs from 09/2010; Performing standard colony PCR using the standard sequenzing primers VF2 and VR. Negative colonies (not containing any binding site pattern, but just religated vector) should show a band at ~290 bp. In parallel, miniprep LB cultures were inocculated using the same, picked colonies. | * Picking of 5 additional colonies of the transformation (binding site patterns) from the plates containing the 200 bp and 400 bp constructs from 09/2010; Performing standard colony PCR using the standard sequenzing primers VF2 and VR. Negative colonies (not containing any binding site pattern, but just religated vector) should show a band at ~290 bp. In parallel, miniprep LB cultures were inocculated using the same, picked colonies. | ||
* although the bands seem too short, the colonies nr. 4, 5 (!), 8 and 10 of the 400 bp band seem positive (gel picture). Minipreps were performed applying the Qiagen Miniprep Kit and were sen for overnight sequenzing (@ GATC). | * although the bands seem too short, the colonies nr. 4, 5 (!), 8 and 10 of the 400 bp band seem positive (gel picture). Minipreps were performed applying the Qiagen Miniprep Kit and were sen for overnight sequenzing (@ GATC). | ||
| - | [[Image:20100811_1.png|thumb|450px| | + | [[Image:20100811_1.png|thumb|450px|center|Result of the colony PCR. The 400 bp binding site pattern samples nr. 4, 5, 8 and 10 were positive and send for sequencing]] |
| - | [[Image:20100811_2.png|thumb|450px| | + | |
| + | [[Image:20100811_2.png|thumb|450px|center|Result of colony PCR. The 400 bp colony-PCRs (nr. 1-10) were loaded on a gel again for further specification of the band length]] | ||
<br /><br /><br /> | <br /><br /><br /> | ||
{{:Team:Heidelberg/Single_Bottom}} | {{:Team:Heidelberg/Single_Bottom}} | ||
Latest revision as of 03:17, 28 October 2010

Binding Site Design - August01/08/2010
02/08/2010
03/08/2010
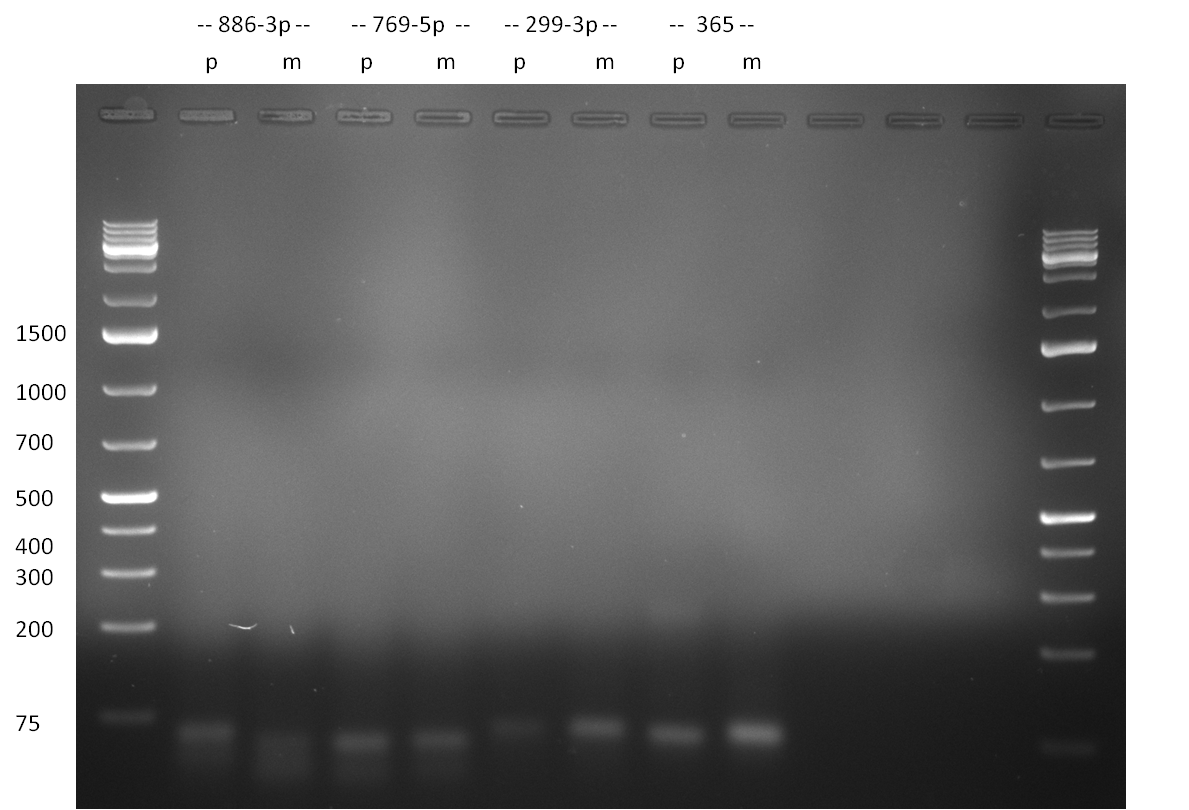
diraPCR for constructing hsa-886-3p binding site patterns the diraPCR was pipetted according to the following protocol in three repeats:
the three 12x repeats (1,2,3) were each split into two (1A, 1B, 2A, 2B, 3A, 3B) and the 25 cycle PCR was performed according to the standard miRACLE PCR protocol after PCR purification the subsequent PCR reaction was pipetted as follows:
04/08/2010
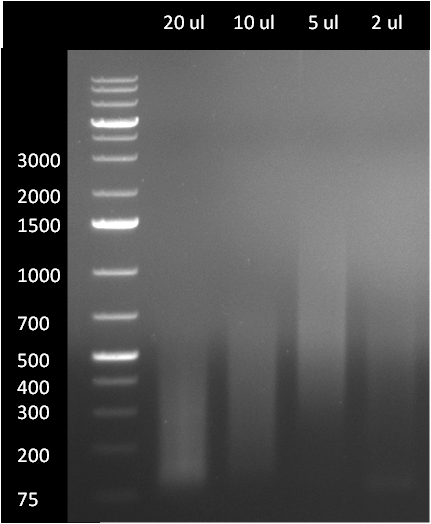
12cycle PCR: 1ul of 5x loading dye + 2.5ul of purified DNA 25cycle PCR: 1ul of 5x loading dye + 5ul of PCR mixture gel map:
1kbLadder - 12x(1)- 12x(2)- 12x(3)- DNAladderMix - 1A - 1B - 2A - 2B - 3A - 3B
05/08/2010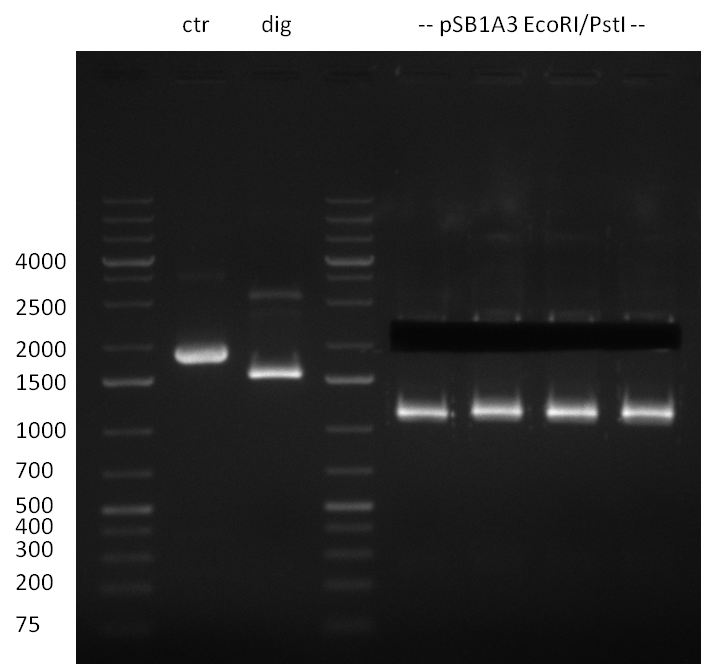 Digestion of the positive clone (previous day) and preperative digestion of vector pSB1A3; Ctr: Test Digestion of Vector pSB1A3, dig: Digestion of positive, no insert is dropping out (negative); 2.1 kb vector band was cut out, but unexpected ~1.2 kb band occured again. Therefor the vector was wasted
Digestion is performed o/n @ 4 °C
06/08/2010
07/08/2010
08/08/2010
09/08/2010
10/08/2010
11/08/2010
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"