Team:Groningen/Hydrophobins
From 2010.igem.org
(→Chaplins) |
(→Chaplins) |
||
| Line 1: | Line 1: | ||
==Chaplins== | ==Chaplins== | ||
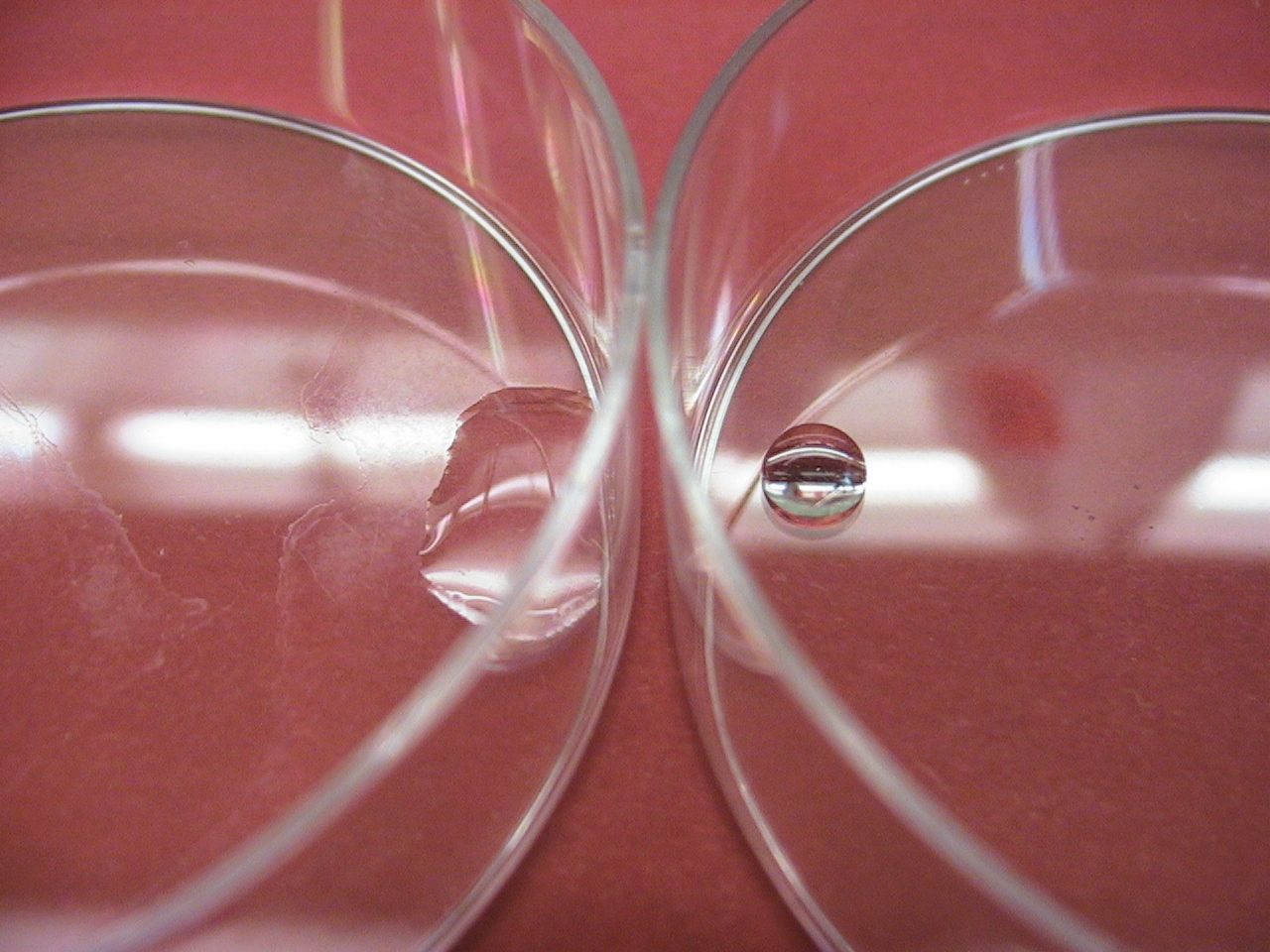
| - | [[Image:Streptomycesdruppel.jpg|right|300px|Credit to M. Gottelt]] | + | [[Image:Streptomycesdruppel.jpg|right|300px|Close up of a Streptomyces coelicolor colony showing its strongly hydrophobic surface. Credit to M. Gottelt]] |
'''Strongly hydrophobic proteins''' | '''Strongly hydrophobic proteins''' | ||
Revision as of 23:34, 24 October 2010
Chaplins
Strongly hydrophobic proteins
In order to provide our Bacillus subtilis biofilm with hydrophobic properties we have turned to highly hydrophobic proteins originating from Streptomyces coelicolor, a soil dwelling bacterium which undergoes morphological differentiation as it goes through different stages in its life cycle, greatly resembling fungal growth. After submerged, vegetative growth aerial hyphae are formed which protrude from the moist substrate. The formation of these aerial hyphae appear to be highly dependent of strongly hydrophobic proteins called chaplins and have previously been described by Claessen et al (2003).
Two subgroups
Chaplins can roughly be categorized into two groups. The first group consists of chaplins A to C and are about 225 amino acids in size. These large chaplins contain two hydrophobic chaplin domains, a hydrophilic region and a cell wall anchor as well as a signaling sequence. The second group includes chaplin D to H and are with around 63 amino acids smaller than the afore mentioned chaplins. Being smaller, they only contain a hydrophobic chaplin domain and a signaling sequence.
Chaplins can roughly be categorized into two groups. The first group consists of chaplins A to C and are about 225 amino acids in size. These large chaplins contain two hydrophobic chaplin domains, a hydrophilic region and a cell wall anchor as well as a signaling sequence. The second group includes chaplin D to H and are with around 63 amino acids smaller than the afore mentioned chaplins. Being smaller, they only contain a hydrophobic chaplin domain and a signaling sequence.
Chaplins are functional amyloids that catalytically assemble into polymer chain forming rod-like structures called amyloid fibers. These fibers are very rigid and hard to break down. These fibers can only be broken when boiled in SDS. They share distinguishing features with the medically important pathogenic amyloid fibers that are characteristic for many neurodegenerative diseases such as Alzheimer's, Huntington's, systemic amyloidosis and the prion diseases.
Claessen, D; Rink, R; de Jong, W et al. 2003. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev 17 1714-1726
Physical properties
Interestingly, purified chaplins can be used to hydrophillically coat normally hydrophobic surfaces such as petri dishes. This is due to their amphipatic nature.
This amphipatic property also gives them oil dispersing abilities as can be seen in the figure below.
 "
"