|
|
| (14 intermediate revisions not shown) |
| Line 122: |
Line 122: |
| | <div class="box_right"> | | <div class="box_right"> |
| | </html> | | </html> |
| - | ==== 18. Labortag 01.06.2010: Modifying MCS of pAAV_MCS vector==== | + | ===6. Labday 03.05.2010=== |
| - | <br>
| + | |
| - | Investigators: Anissa, Adrian, Bea, Chris W., Hanna, Patrick, Volker, Sven <br>
| + | |
| | | | |
| - | '''Oligos received from Sigma-Aldrich''' <br>
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Theoretical cloning</b></p>==== |
| - | (right ITR of pAAV_MCS, left ITR of pAAV_MCS and MCS RFC25 for pAAV)
| + | |
| - | <br>
| + | |
| - | *Hybrization of received oligos: MCS RFC25 for pAAV (forward) and MCS RFC25 for pAAV (reverse)
| + | |
| - | *Centrifuge tubes prior to open tubes (13.000 rpm, 30 sec)
| + | |
| - | **MCS RFC25 for pAAV (forward): Add 92µL Millipore H<sub>2</sup>O (Volume on obtained sheet)
| + | |
| - | **MCS RFC25 for pAAV (reverse): Add 394 µL Millipore H<sub>2</sup>O (Volume on obtained sheet)
| + | |
| - | *Vortex the resuspended DNA
| + | |
| - | *Make aliquots of both Oligos (1:10): 10 µL Oligo + 90 µLH<sub>2</sup>O (final volume usually 100 µl)
| + | |
| - | *Mix together(into PCR-tube):
| + | |
| - | <br>
| + | |
| | | | |
| - | {| border="1"
| + | <p><b>Investigators: Adrian, Hanna, Bea, Patrick, Chris W. </b></p><br> |
| - | | align="right" | '''Volume/µL''' ||align="right"| '''solution'''
| + | |
| - | |-
| + | |
| - | | align="right" | 10 (1:10)||align="right"|Oligo 1: MCS RFC25 for pAAV (forward)
| + | |
| - | |-
| + | |
| - | | align="right" |10||align="right"|Oligo 2: MCS RFC25 for pAAV (reverse)
| + | |
| - | |-
| + | |
| - | | align="right" |4||align="right"| 100mM TrisHCl pH8
| + | |
| - | |-
| + | |
| - | | align="right" |8||align="right"|5mM MgCl2
| + | |
| - | |-
| + | |
| - | | align="right" |8||align="right"| H20
| + | |
| - | |}
| + | |
| - | <br> | + | |
| - | * Program: ORIGAMI 1 modified for long oligos:
| + | |
| - | ** 1 99°C 7’
| + | |
| - | ** 2 99°C 1’
| + | |
| - | ** -1°C R=0.3 °/s
| + | |
| - | ** Goto 2 rep 74
| + | |
| - | ** Hold 4°C
| + | |
| - | <br> | + | |
| - | *While hybridization of oligos is performed, digestion of pAAV_MCS vector can be conducted <br>
| + | |
| - | following standard protocol for cloning.
| + | |
| - | <br> | + | |
| - | *Title: Ligation MCS_Oligo with pAAV_MCS
| + | |
| - | *Plasmid: pAAV_MCS
| + | |
| - | *Buffer used: 3
| + | |
| - | *BSA: Yes
| + | |
| - | *Measure DNA-concentration with Nanodrop
| + | |
| - | *DNA-Concentration:260 ng/uL
| + | |
| - | *Restriction-enzyms used: http://www.neb.com/nebecomm/DoubleDigestCalculator.asp
| + | |
| - | Enzyme1 (Nr. Lab: 152): ClaI
| + | |
| - | Enzyme2 (Nr. Lab: 15): BglII
| + | |
| - | <br> | + | |
| - | *Digestion components :
| + | |
| | | | |
| - | {| border="1"
| + | |
| - | | '''components''' || align="right" | '''pAAV_MCS'''
| + | There are several matters to do in theoretical cloning. The modularization/modification of the stratagene plasmids and the enzymes. :<br> |
| - | |-
| + | |
| - | | DNA || align="right" | 4
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |3
| + | |
| - | |-
| + | |
| - | | Buffer 3 (10x)|| align="right" |3
| + | |
| - | |-
| + | |
| - | |Enzyme: ClaI (no.Lab:152)|| align="right" |2
| + | |
| - | |-
| + | |
| - | |Enzyme: BglII (no.Lab:15)|| align="right" |1
| + | |
| - | |-
| + | |
| - | |H<sub>2</sup>O|| align="right" |17
| + | |
| - | |-
| + | |
| - | |'''Total volume'''|| align="right" |<b>30</b>
| + | |
| - | |}
| + | |
| - | *Incubate for 1,5 h at 37°C
| + | |
| | <br> | | <br> |
| - | *1% Agarose gel
| + | 1. Cap-Gen: |
| - | **1% agarose gel was prepared, gel ran for 45 minutes( first: 90V, after 15 minutes: 115 V) | + | * delete the PstI-restriction site |
| | + | * insert the antibody fragment (targeting): sequence? in which part of VP1? (Patrick) |
| | + | * add prefix & suffix |
| | + | * disable binding of Heparan Sulphat Proteoglycan |
| | <br> | | <br> |
| - | *Amount of loading dye added
| + | 2. Rep-Gen: |
| - | {| border="1"
| + | * delete EcoRI (2x) and PstI (2x) |
| - | |<b>sample/µL </b>||align="right"| '''loading dye/µL'''
| + | |
| - | |-
| + | |
| - | | marker: 8 ||align="right"|contains loading dye
| + | |
| - | |-
| + | |
| - | |pAAV_MCS: 24 ||align="right"|6
| + | |
| - | |-
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| - | *Expected size of fragments | + | 3. ITRs: |
| - | | + | * NotI and PstI (in sequence?) flank the ITRs: its the question if we can/must delete them? |
| - | {| border="1"
| + | * where exactly starts and ends the sequence (Patrick)? |
| - | | '''sample''' ||align="right"| '''expected size'''
| + | * it should be checked up if there is a possibility to use all of the three ITRs. |
| - | |-
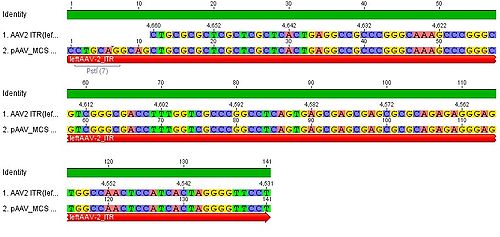
| + | * we blasted the ITR (left) of the MCS vektor (Stratagene). we got a 92% "Coverage" with the AAV2 genom (to 100%). from the alignment (Stratagene ITR with ITR of the AAV2 genom) we got:<br> |
| - | | align="right" | pAAV_MCS: cut with ClaI and BglII||align="right"|4580 bp
| + | [[File:Freiburg10 ITR(left)-Stratagene vs. ITR AAV2.jpg|500x500px|]] |
| - | |-
| + | You can see, that the ITR sequence beginns 4 bp after the PstI restriction site. thereupon we did a secundary structure analyse (www.dinamelt.bioinfo.rpi.edu), with the following structurewe got: <br> |
| - | |}
| + | [[Media:Freiburg10_AAV2ITR(left)nachBlast.pdf]] <br> |
| | + | conclusion: the big loop of the secondary structure dosent change if we delete the PstI restriction site (cf. with other uploadet secondary structures), it should be considered that we can't add random bp that could affect the secundary structure. <br> |
| | + | <b>To do: which bp, which sequence could/can be insertet? cf. the genome of AAV2</b> <br> |
| | <br> | | <br> |
| - | | + | 4. MCS: |
| - | ==== 19. Labortag 02.06.2010: Oligos (NotI)====
| + | * replacement through the iGEM-MCS |
| - | | + | * where is the beginning and ending of the MCS in the Vector? ß-globin function and sequence (search for literature and patents, which are denoted in the AAV-Helper-Free-System Manual) |
| - | Investigators: Adrian, Bea, Chris W., Hanna, Anissa<br>
| + | * in this context it would be also important to find out where the beta-Globulin-Intron exactly starts or rather we can cut out the MCS. |
| - | <br>
| + | |
| - | '''Practical work:''' <br>
| + | |
| - | Control plate contained no clones. :)
| + | |
| - | 4 colonies were picked and grown @ 37°C over night.
| + | |
| | <br> | | <br> |
| - | '''Theoretical work:'''<br>
| + | 5. enzymes: |
| - | Oligos for site directed mutagenesis of the NotI restriction sites in pAAV_MCS (ITRs) were designed:[[File:Freiburg10 NotI ITR Oligos.pdf]]
| + | * Thymidinkinase: find informations. TK30 (Bea), SR39 (Hanna) |
| | + | * Cytosindeaminase: find informations (Adrian) |
| | <br> | | <br> |
| - | '''Sponsoring work:''' <br>
| + | in addition we downloadet, addet and anotated the sequence of the pHelper plasmid of stratagene in Geneious (see "Constructs"). |
| - | Sponsoring letter was adapted for Quiagen.
| + | |
| | | | |
| - | ====20. Labortag 03.06.2010: pAAV_RFC25_MCS -> problem====
| |
| | | | |
| - | Investigators: Anissa, Bea, Melanie, Christian L.
| + | insert the antibody fragment (targeting): sequence? in which part of VP1? (Patrick) |
| - | <br>
| + | |
| | | | |
| - | '''Comment''': Continue with Mini-Prep and test digestion of pAAV_RFC25_MCS <br>
| + | ===7. Labday 07.05.2010=== |
| - | Mini-Prep and test digestion have been performed: <br>
| + | |
| | | | |
| - | <span style="color:red; font-weight:bold;">Problem</span>: Designed oligos (MCS_RFC25) for altering the MCS of the pAAV_MCS vector cannot be used. <br> Two startcodons are in the MCS. | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Protocolls</b></p>==== |
| - | [[File:Oligo RFC25 MCS.jpg|800px|thumb|left| Oligo MCS_RFC25: contains 2 Startcodons. the "wrong" Codon is the first ATG which results in peptide chain with 30 aa. The second ATG is the right ATG which is right before the Gene of Interest.]]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | The two startcodons are not in the same open reading frame (ORF). Therefore two proteins will be produced. The Gene of Interest and the short peptide (30 aa).<br>
| + | |
| - | The idea of the oligos was to ligate the oligos into the pAAV_MCS vector. The oligos contained two overhangs which correspond to the sequences of the two restriction sites ClaI and BglII: <br>
| + | |
| - | The pAAV_MCS vector was digested with ClaI and BglII and then we ligated the oligo and the vector. Problem was that we did not notice that the overhang of ClaI and the sequence of EcoRI of the MCS_RFC25 resulted in another ATG startcodon.
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''Possible Solutions''':
| + | |
| - | <ul>
| + | |
| - | # first: modify MCS with ordered oligos of Sven (shorter MCS which cannot be used for pEX)and clone mVenus
| + | |
| - | # Perform site-directed-mutagenesis (QuikChange from Stratagene)
| + | |
| - | # Order new MCS-oligos and consider that '''no''' new ATG is produced. For example: add another base between ClaI overhang and EcoRI sequence. -----AT'''X'''G---- '''This solution is the more possible one we are going to perform.'''
| + | |
| - | </ul>
| + | |
| - | <br>
| + | |
| - | <span style="color:blue; font-weight:bold;">Practical work</span>
| + | |
| - | <br>
| + | |
| - | <ul>
| + | |
| - | *Preparing four glycerol stocks (2:1)
| + | |
| - | **numbers: B4 - B7 (for details see nomenclature)
| + | |
| - | **stored in -80°C, Box 1
| + | |
| - | </ul>
| + | |
| - | *<b>MiniPrep</b>
| + | |
| - | **Nanodrop concentrations
| + | |
| - | <br> | + | |
| - | {| border="1"
| + | |
| - | | align="right" | '''Sample''' ||align="right"| '''Concentration/ng*µl-1'''
| + | |
| - | |-
| + | |
| - | | align="right" | P11 ||align="right"|340,5
| + | |
| - | |-
| + | |
| - | | align="right" | P12 ||align="right"|364,0
| + | |
| - | |-
| + | |
| - | | align="right" | P13 ||align="right"|358,5
| + | |
| - | |-
| + | |
| - | | align="right" | P14 ||align="right"|284,4
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | *Test digestion
| + | |
| - | <br>
| + | |
| - | {| border="1"
| + | |
| - | | Components || align="right" |Volume µl ||align="right"| Mastermix µl
| + | |
| - | |-
| + | |
| - | | DNA || align="right" | 800 || align="right" | --
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" | 1,5 || align="right" | 7,5
| + | |
| - | |-
| + | |
| - | | Buffer No.2 (10x)|| align="right" | 1,5 || align="right" | 7,5
| + | |
| - | |-
| + | |
| - | |Enzyme 1 (no.Lab:45) Nde I || align="right" | 0,5 || align="right" | 2,5
| + | |
| - | |-
| + | |
| - | |Enzyme 2 (no.Lab:71) Spe I || align="right" | 0,5 || align="right" | 2,5
| + | |
| - | |-
| + | |
| - | |H<sub>2</sup>O|| align="right" | variable || align="right" | --
| + | |
| - | |-
| + | |
| - | |'''Total volume '''|| align="right" | 15 || align="right" | 20
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | {| border="1"
| + | |
| - | | Sample || align="right" | Volume/ µl ||align="right"| H<sub>2</sup>O / µl
| + | |
| - | |-
| + | |
| - | | P11 || align="right" | 2,3 ||align="right"| 8,7
| + | |
| - | |-
| + | |
| - | | P12 || align="right" | 2,2 ||align="right"| 8,8
| + | |
| - | |-
| + | |
| - | | P13 || align="right" | 2,2 ||align="right"| 8,8
| + | |
| - | |-
| + | |
| - | | P14 || align="right" | 2,8 ||align="right"| 8,2
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | *Incubation: 1,5 h
| + | |
| - | <br>
| + | |
| - | <li>Agarose-Gel
| + | |
| | | | |
| - | *Materials
| + | <p><b>Investigators: Anissa, Kerstin </b></p><br> |
| - | 0,5 g Agarose, 50 ml TAE, 3µl GELRED, at 100 Volt, running time: 45 minutes
| + | |
| | | | |
| | + | * adaption and extension of the standart prorcolls (Cloning for Pro's) |
| | + | * we startet to create a protokoll-mask for the praktical-cloning (short version for labwork) |
| | | | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | ===8. Labday 10.05.2010 === |
| - | !Sample
| + | |
| - | !Sample/µl]
| + | |
| - | !Loading dye (5x/6x)/µl
| + | |
| - | !Expected size 1 (Geneious)
| + | |
| - | !Expected size 2 (Geneious)
| + | |
| - | |--
| + | |
| - | |P11
| + | |
| - | |15 µl
| + | |
| - | |3 µl
| + | |
| - | |3677 bp
| + | |
| - | | 974 bp
| + | |
| - | |--
| + | |
| - | |P12
| + | |
| - | |15 µl
| + | |
| - | |3 µl
| + | |
| - | |3677 bp
| + | |
| - | | 974 bp
| + | |
| - | |--
| + | |
| - | |P13
| + | |
| - | |15 µl
| + | |
| - | |4 µl
| + | |
| - | |3677 bp
| + | |
| - | | 974 bp
| + | |
| - | |--
| + | |
| - | |P14
| + | |
| - | |15 µl
| + | |
| - | |4 µl
| + | |
| - | |3677 bp
| + | |
| - | | 974 bp
| + | |
| - | |--
| + | |
| | | | |
| - | |}
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Stock solutions</b></p>==== |
| - | {| align=right
| + | |
| - | |}
| + | |
| | | | |
| | + | <p><b>Investigators: Adrian, Chris W., Chris L., Bea, Achim, Patrick, Hanna, (Sven)</b></p><br> |
| | + | The following stock solutions were prepared: <br> |
| | + | 1. Antibiotics: |
| | + | * Ampicillin: 2 g Ampicillin were dissolved in 20 mL ethanol (70%), filled into 2 mL tubes and stored at -20°C. |
| | + | * Chloramphenicol: 0.5 g Chloramphenicol were dissolved in 20 mL ethanol (70%), filled into 2 mL tubes and stored at the -20°C. |
| | + | * Kanamycin: 1 g Kanamycin was dissolved in 20 mL multipore-H2O, sterilized by filtration and filled into 2 mL tubes and stored at the -20°C. |
| | + | * Tetracyclin: 0.5 g Tetracyclin were dissolved in 20 mL ethanol (70%) and filled into 2 mL tubes. The tubes were wrapped with aluminium foil (light sensitive!) and stored at -20°C. |
| | + | 2. ITPG solution (1 M): |
| | + | * ~ 4.766 g ITPG were dissolved in 20 mL multipore-H2O, sterilized by filtration, filled in 2 mL tubes and stored at -20°C. |
| | + | 3. DYT (5 litres) |
| | + | * 80 g Bactotrypton, 50 g Bactoyeast, 25 g NaCl were weight out. |
| | + | * 2 L multipore-H2O were added |
| | + | * after mixing, multipore-H2O was added -> endvolume 5 litres |
| | + | * medium was filled into flask and was autoclaved |
| | + | 4. Glycerol: |
| | + | * Glycerol was filled into a flask and was then autoclaved |
| | | | |
| - | {| border="1"
| + | '''To do: register at Mr. Gene!!!''' |
| - | |
| + | |
| - | !Marker
| + | |
| - | !Sample P11 /18 µl
| + | |
| - | !Sample P12 /18 µl
| + | |
| - | !Sample P13 /19 µl
| + | |
| - | !Sample P14 /19 µl
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | | 1
| + | |
| - | | 3
| + | |
| - | | 5
| + | |
| - | | 7
| + | |
| - | | 9
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | | + | |
| - | '''Results of agarose-gel:''' | + | |
| | <br> | | <br> |
| - | <ul>
| |
| - | *Expected fragments of 3677 bp and 974 bp can be cerified. The insertion of the RFC25_MCS has been inserted. <br>
| |
| - | For further verification the insert has to be sequenced has to be conducted (to do for 04.06.2010). <br>
| |
| - | </ul>
| |
| - | <br>
| |
| - |
| |
| - | <span style="color:blue; font-weight:bold;">Picking clones of Thymindinkinase of Amor</span>
| |
| - | **5 clones of the XL-1 Blue colonies containing the plasmid '''pUB6/HV5/His6 with the thymidinkinase''' have been picked from LBamp-agarplates
| |
| - | **1 clone of the XL-1 Blue colonies containing the plasmid '''pUB6/HV5/His6 without the thymidinkinase (control) has been picked from LBamp-agarplates
| |
| - | **all clones have been inoculated in 10 mL LB containing 10 µL Amp. Incubation: 37°C over-night.
| |
| - | **'''to do: Mini-Prep of pUB6/HV5/His6 with the thymidinkinase and pUB6/HV5/His6 without the thymidinkinase (control)'''
| |
| - | <br>
| |
| - | <span style="color:blue; font-weight:bold;">Idea</span>
| |
| - | <ul>
| |
| - | *Insertion of Kozak consensus sequence before MCS to enhance gene expression (cloning consideration in Stratagene manual)
| |
| - | ** New RFC standard with Kozak sequence for eucaryotes??
| |
| - |
| |
| - | ====21. Labortag 04.06.2010: DKFZ plasmid Retrafos, TK/GMK Mini-Prep====
| |
| | | | |
| - | Investigators: Adrian, Bea, Chris W., Hanna<br>
| + | ===9. Labday 17.05.2010=== |
| - | <p style="font-size:15px; font-weight: bold; color: green;"><u>DKFZ</u></p>
| + | |
| | | | |
| - | '''Comments''': Plasmids of PD Kleinschmidt of the DKFZ arrived. The DNA was dried on a whatman paper.<br>
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>β-Globin</b></p>==== |
| - | Plasmids received: <br>
| + | |
| | | | |
| - | *'''pXX6''' alle Adenovirus Helfer Gene ohne AAV Gene; Plasmid von J. Samulski; evtl. Anfragen für Benutzererlaubnis
| + | <p><b>Investigators: Bea, Chris W., Patrick, Hanna (and instructors)</b></p><br> |
| - | *'''pKEX-2XL.Rep40''' Expressionsplasmide für das Rep Proteine 40
| + | |
| - | *'''pKEX-2XL.Rep 52''' Expressionsplasmide für das Rep Proteine 52
| + | |
| - | *'''pKEX-2XL.Rep 68''' Expressionsplasmide für das Rep Proteine 68
| + | |
| - | *'''pKEX-2XL.Rep 78''' Expressionsplasmide für das Rep Proteine 78
| + | |
| - | *'''pCMV-VP(HS)''' Expressionsplasmid der drei VP Proteine in Kombination (assemly kompetent
| + | |
| - | *'''pKEX-VP1''' Expressionsplasmide für die einzelnen VP (cap) Proteine (VP1, VP2 und VP3) vorsicht: sind alleine nicht assembly kompetent; Ruffing et al 1992; Steinbach et al. 1997;
| + | |
| - | *'''pKEX-VP2'''Expressionsplasmide für die einzelnen VP (cap) Proteine (VP1, VP2 und VP3) vorsicht: sind alleine nicht assembly kompetent; Ruffing et al 1992; Steinbach et al. 1997;
| + | |
| - | *'''pKEX-VP3'''Expressionsplasmide für die einzelnen VP (cap) Proteine (VP1, VP2 und VP3) vorsicht: sind alleine nicht assembly kompetent; Ruffing et al 1992; Steinbach et al. 1997;
| + | |
| - | *'''pTRUF_CMV_eGFP'''Einzelstrang Vektor zur Expression von eGFP
| + | |
| - | *'''dsAAV_CMV_eGFP'''"self complementary" Expressionsvektor von X. Xiao; evtl. anfragen wegen Benutzererlaubnis
| + | |
| - | *'''pTAV2 (gesamtes AAV Genom im "blue script" Vektor)
| + | |
| - | *'''pDG''' komplettes Helferplasmid zur Vektorherstellung; Grimm et al. 1998
| + | |
| | <br> | | <br> |
| - | *In order to obtain the DNA following steps have been performed:
| + | We blasted the sequence of the β-globin and additional nukleotides „vorne und hintendran“. |
| - | **cut out the spot where the DNA is spotted with a clean scalpel (note: scalpel should not have any contamination).
| + | We found only 70% coverage with the human β-globin-intron add. some parts of the exon 3. |
| - | **put a 0,5 mL Eppi in a 1,5 mL Eppi. Put little holes in the smaller eppi.
| + | For this reason we think that the pAAV_MCS annotation of the β-Globin-intron of Stratagene is too generously.<br> |
| - | **transfer Whatman paper into Eppi
| + | In addition the alignment of the human ß-globin showed that only parts of the intron 2 (5’) and exon 3 are integratet into the vector. |
| - | **add 50 µL TE-EF (Redissolving buffer) to whatman paper and wait 15 minutes
| + | We assume that the informations from Stratagene are not total correctly ( intron flanket by the splicedonors and the acceptor-sequence). |
| - | **centrifuge eppis at 2000 rpm, 10 minutes
| + | We blasted the sequence between the CMV-promoter and the origin beginning of the ß-globin intron. |
| - | *Transformation with obtained plasmids was performed.
| + | We found a 98,8% coverage with a synthetic CMV-promoterconstruct ( 1 nucleotide difference). |
| - | </ul>
| + | Literature: „Diverse plasmid DNA vectors by directed molecular evolution of cytomegalovirus promoters. (Wright A. et al.)“<br> |
| - | <br>
| + | →The question arises if we can omit the ß-globin (because the exact function is unknown). |
| - | <p style="font-size:15px; font-weight: bold; color: green;"><u>Fusion Enzyme: Thymidinkinase/Guanylate Kinase (TK/GMK)</u></p>
| + | For this we should contact various Companys (GeneArt, DNA2.0, Mr. Gene,…) to get more information. |
| - | <p style="font-size:12px; font-weight: bold; color: blue;"><u>Plasmid Mini-Prep</u></p>
| + | In addition we could test the expression with and without ß-globin. |
| - | *experiment date: 04.06.2010 ; time: 3,5h
| + | |
| - | *name of investigator: Adrian, Bea, ChrisW.,Hanna
| + | |
| - | *new vector name: pUB_V5_His6 + TK/GMK (fusionenzyme)
| + | |
| - | <br />
| + | |
| - | <u>Glycerol Stocks</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4''' ||align="left"| '''Clone 5'''||align="left"|'''Control'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| XL-1 Blue ||align="left"| XL-1 Blue ||align="left"| XL-1 Blue ||align="left"| XL-1 Blue ||align="left"| XL-1 Blue ||align="left"| XL-1 Blue
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pUB_V5_His6 + TK/GMK ||align="left"| pUB_V5_His6 + TK/GMK ||align="left"| pUB_V5_His6 + TK/GMK ||align="left"| pUB_V5_His6 + TK/GMK ||align="left"| pUB_V5_His6 + TK/GMK ||align="left"| pUB_V5_His6 + TK/GMK
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 04.06.2010 ||align="left"| 04.06.2010 ||align="left"| 04.06.2010 ||align="left"| 04.06.2010 ||align="left"| 04.06.2010 ||align="left"| 04.06.2010
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| B8 ||align="left"| B9 ||align="left"| B10 ||align="left"| B11 ||align="left"| B12 ||align="left"| B13
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| - | <u>Given Plasmid-Number</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4''' ||align="left"| '''Clone 5''' ||align="left"| '''Control'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| P15 ||align="left"| P16 ||align="left"| P17 ||align="left"| P18 ||align="left"| P19 ||align="left"| P20
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <p style="font-size:12px; font-weight: bold; color: blue;"><u>Nanodrop concentration</u></p>
| + | |
| - | *Plasmid
| + | |
| - | **Given Plasmid-Number: P15; DNA concentration: 493,7 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P16; DNA concentration: 464,4 ng/µL;
| + | |
| - | **Given Plasmid-Number: P17; DNA concentration: 445,6 ng/µL;
| + | |
| - | **Given Plasmid-Number: P18; DNA concentration: 562,9 ng/µL;
| + | |
| - | **Given Plasmid-Number: P19; DNA concentration: 528,1 ng/µL;
| + | |
| - | **Given Plasmid-Number: P20; DNA concentration: 499,9 ng/µL;
| + | |
| - | <br /> | + | |
| - | '''Comments:'''A Plasmid-Mini Prep with the received Fusionenzyme Thymidinkinase/Guanylate Kinase (TK/GMK)from Amor has been performed.<br>
| + | |
| - | The DNA will be sent to GATC for sequencing.
| + | |
| - | *pUB6_V5_His6 - clone 1 (P15) (3 µL added to 27µL H20) has been sent to GATC.
| + | |
| - | *Expected results: Saturday
| + | |
| | | | |
| - | <br />
| |
| | <br> | | <br> |
| | | | |
| - | ====22. Labortag 05.06.2010: Insertion of iGEM expression parts into pAAV_MCS==== | + | ===10. Labortag 18.05.2010=== |
| | | | |
| - | Investigators: Adrian, Bea, Melanie, Hanna<br>
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>pCMV_mVenus_YFP</b></p>==== |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Hybridization:</p> | + | |
| - | *Hybridization of received oligos: iGEM expression parts (RFC25 without EcoRI, NotI)
| + | |
| - | *Prior to opening the tubes, they were centrifugated at 13.000 rpm for 30 sec.
| + | |
| - | **expression part MCS_for (charge-no: ST00114065): 108 µL Millipore H<sub>2</sup>O (Volume on obtained sheet)were added.
| + | |
| - | **expression part MCS_for (charge-no: ST00114066): 165 µL Millipore H<sub>2</sup>O (Volume on obtained sheet)were added.
| + | |
| - | *Resuspended DNA was vortexted.
| + | |
| - | *Aliquots of both Oligos (1:10) were prepared: 10 µL Oligo + 90 µL H<sub>2</sup>O (final volume usually 100 µl).
| + | |
| - | *Mix together(into PCR-tube):
| + | |
| - | <br>
| + | |
| - | {| border="1"
| + | |
| - | | align="right" | '''Volume/µL''' ||align="right"| '''solution'''
| + | |
| - | |-
| + | |
| - | | align="right" | 10 (1:10)||align="right"|Oligo 1: expression part MCS_for (charge-no: ST00114065)
| + | |
| - | |-
| + | |
| - | | align="right" |10 (1:10)||align="right"|Oligo 2: expression part MCS_for (charge-no: ST00114066)
| + | |
| - | |-
| + | |
| - | | align="right" |4||align="right"| 100 mM TrisHCl pH8
| + | |
| - | |-
| + | |
| - | | align="right" |8||align="right"|5 mM MgCl2
| + | |
| - | |-
| + | |
| - | | align="right" |8||align="right"| H20
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | * Program: ORIGAMI 1 modified for long oligos:
| + | |
| - | ** 1 99°C 7’
| + | |
| - | ** 2 99°C 1’
| + | |
| - | ** -1°C R=0.3 °/s
| + | |
| - | ** Goto 2 rep 74
| + | |
| - | ** Hold 4°C
| + | |
| - | <br>
| + | |
| - | *While hybridization of oligos was performed, digestion of pAAV_MCS vector was conducted <br>
| + | |
| - | following standard protocol for cloning.
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Digestion:</p>
| + | |
| - | *Title: Ligation iGEM expression parts (="iGEM-MCS") with pAAV_MCS
| + | |
| - | *Plasmid: pAAV_MCS
| + | |
| - | *Buffer used: 3
| + | |
| - | *BSA: Yes
| + | |
| - | *DNA-Concentration: 260 ng/uL
| + | |
| - | *Restriction-enzyms used:
| + | |
| - | Enzyme1 (Nr. Lab: 152): ClaI
| + | |
| - | Enzyme2 (Nr. Lab: 15): BglII
| + | |
| - | <br>
| + | |
| - | *Digestion components :
| + | |
| | | | |
| - | {| border="1"
| |
| - | | '''components''' || align="right" | '''pAAV_MCS'''
| |
| - | |-
| |
| - | | DNA || align="right" | 5.8 µL
| |
| - | |-
| |
| - | | BSA (10x) || align="right" |3 µL
| |
| - | |-
| |
| - | | Buffer 3 (10x)|| align="right" |2 µL
| |
| - | |-
| |
| - | |Enzyme: ClaI (no.Lab:152)|| align="right" |2 µL
| |
| - | |-
| |
| - | |Enzyme: BglII (no.Lab:15)|| align="right" |1 µL
| |
| - | |-
| |
| - | |H<sub>2</sup>O|| align="right" |16.2 µL
| |
| - | |-
| |
| - | |'''Total volume'''|| align="right" |<b>30 µL</b>
| |
| - | |}
| |
| - | *Mixture was incubated for 1,5 h at 37°C.
| |
| - | <br>
| |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Agarose-Gel:</p>
| |
| - | *1% agarose gel was prepared, gel ran for 45 minutes(110 V)
| |
| - | <br>
| |
| - | *Amount of loading dye added
| |
| - | {| border="1"
| |
| - | |<b>sample/µL </b>||align="right"| '''loading dye/µL'''
| |
| - | |-
| |
| - | | marker: 8 ||align="right"|contains loading dye
| |
| - | |-
| |
| - | |pAAV_MCS: 30 ||align="right"|6 (6x loading dye)
| |
| - | |-
| |
| - | |}
| |
| - | <br>
| |
| - | *Expected size of fragments
| |
| | | | |
| - | {| border="1"
| + | <p><b>Investigator: Bea, Chris W., Patrick, Hanna, Anissa, Kerstin, Adrian (und Instructors)</b></p> |
| - | | '''sample''' ||align="right"| '''expected size'''
| + | |
| - | |-
| + | |
| - | | align="right" | pAAV_MCS: cut with ClaI and BglII||align="right"|4580 bp
| + | |
| - | |-
| + | |
| - | |}
| + | |
| | | | |
| | | | |
| | + | Theoretical cloning with Geneious of pCMV-MCS + pGA14_mVenus_YFP --> <b>pCMV_mVenus_YFP</b> |
| | + | <ul> |
| | + | <li>Enzyme set: RFC 25 (iGEM) |
| | + | <li> digest pGA14_mVenus_YFP (insert) with XbaI and PstI: mVenus_YFP_cut_XbaI+PstI |
| | + | <li> digest pCMV_MCS (vector) with XbaI and PstI: pCMV_MCS_cut_XbaI+PstI |
| | + | <li> ligate mVenus_YFP_cut_XbaI+PstI with pCMV_MCS_cut_XbaI+PstI |
| | + | <br> |
| | + | [[File:Freiburg10 pCMV MCS mVenus YFP.png|500x500px|]] |
| | </ul> | | </ul> |
| - | <br>
| |
| - | <br>
| |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Gelextraction:</p>
| |
| - | <br />
| |
| - | Gel measurement:
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | align="left" | '''Sample''' ||align="left"| '''weight'''
| |
| - | |-
| |
| - | | align="left" |pAAV_MCS ||align="left"| 60 mg
| |
| - | |-
| |
| - | |}
| |
| - | *Gelextraction was performed following standard protocol.
| |
| - | *DNA-concentrations were meassured: pAAV_MCS 18.2 ng/µL, Oligos: 136.7 ng/µL -> 1:10 dilution was prepared: 13.67 ng/µL
| |
| - | <br /><br />
| |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Ligation:</p>
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | align="left" | ||align="left"| '''iGEM-MCS''' ||align="left"| '''pAAV_MCS'''
| |
| - | |-
| |
| - | | align="left" | '''Volume/µl''' ||align="left"| 0.4 ||align="left"| 8.6
| |
| - | |}
| |
| - | <br />
| |
| - | Trafo was performed (using XL1B cells) following standard protocol.
| |
| - | <br>
| |
| - | <br>
| |
| | | | |
| - | ====23. Labortag 07.06.2010: Mini-Preps (Kleinschmidt-plasmids and pAAV_iGEM-MCS)==== | + | ===11. Labortag 19.05.2010=== |
| - | investigators: Achim, Kira,Jessy, Chris W., Hanna, Adrian, Bea
| + | |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>pAAV_iGEM-MCS</u></p>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;"><u>Plasmid Mini-Prep</u></p>
| + | |
| - | *experiment date:07.06.2010; time: whole day
| + | |
| - | *name of investigator: Achim, Kira,Jessy, Chris W., Hanna, Adrian, Bea
| + | |
| | | | |
| - | <br />
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Cloning of pCMV-MCS + pGA14_mVenus_YFP --> <b>pCMV_mVenus_YFP</b></p>==== |
| - | <u>Glycerol Stocks</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pAAV_iGEM-MCS ||align="left"| pAAV_iGEM-MCS||align="left"| pAAV_iGEM-MCS||align="left"| pAAV_iGEM-MCS
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 07.06.2010 ||align="left"| 07.06.2010||align="left"| 07.06.2010||align="left"| 07.06.2010
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| - ||align="left"| B27 ||align="left"| B28||align="left"| B29
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <u>Given Plasmid-Number</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| - ||align="left"| P34.2 ||align="left"| P34.3||align="left"| P34.4
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Test digestion</p> | + | |
| - | *buffer used: 4 ; Restriction-enzymes used: Enzyme 1 (no. Lab:___) AgeI ; Enzyme 2 (no.Lab:___) NdeI
| + | |
| - | *Plasmid
| + | |
| - | **Given Plasmid-Number: P34.2; DNA concentration: 433.78 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P34.3; DNA concentration: 408.48 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P34.4; DNA concentration: 409.80 ng/µL ;
| + | |
| | | | |
| - | <br /> | + | <p><b>Investigators: Adrian, Bea, Chris W., Hanna, Patrick</b></p> |
| - | '''Comments:'''Clone no.1 was dismissed...
| + | |
| - | <br /> | + | |
| - | <br />
| + | |
| - | '''Test digestion:'''
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | '''Components''' ||align="left"| '''Volume/µL''' ||align="left"| '''Mastermix'''
| + | |
| - | |-
| + | |
| - | | align="left" | DNA (clone 2)||align="left"| 1000 ng ||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | BSA (10x) ||align="left"| no ||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | Buffer no. 4 (10x) ||align="left"| 1.5 µL||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 1 (no. Lab: ) AgeI ||align="left"| 0.75 µL ||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 2 (no. Lab: ) NdeI ||align="left"| 0.5 µL||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | H<sub>2</sup>O ||align="left"| variable ||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | '''Total volume''' ||align="left"| '''15 µL''' ||align="left"| -
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| - | {| border="1"
| + | |
| - | | Sample || align="right" | Volume sample/ µl ||align="right"| Volume H<sub>2</sup>O / µl
| + | |
| - | |-
| + | |
| - | | P34.2 || align="right" |2.3 ||align="right"| 9.95
| + | |
| - | |-
| + | |
| - | | P34.3 || align="right" | 2.4 ||align="right"| 9.85
| + | |
| - | |-
| + | |
| - | | P34.4 || align="right" | 2.4||align="right"| 9.85
| + | |
| - | |-
| + | |
| | | | |
| - | |-
| + | <b>Digestion</b> |
| - | |}
| + | |
| - | *Incubation: 45 min, 37°C
| + | |
| - | <br /> | + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Agarose-Gel:</p>
| + | |
| - | <br />
| + | |
| - | 0.5 g Agarose, 50 ml TAE (1%), 3 µL GELRED (3-6µl), at 110 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample/µl]
| + | |
| - | !Loading dye (5x/6x)/µl
| + | |
| - | !Expected size 1 (Geneious)
| + | |
| - | !Expected size 2 (Geneious)
| + | |
| - | |--
| + | |
| - | |P34.2
| + | |
| - | |15 µl
| + | |
| - | |3 µl
| + | |
| - | |3686 bp
| + | |
| - | |951 bp
| + | |
| - | | + | |
| - | |--
| + | |
| - | |P34.3
| + | |
| - | |15 µl
| + | |
| - | |3 µl
| + | |
| - | |3686 bp
| + | |
| - | |951 bp
| + | |
| - | | + | |
| - | |--
| + | |
| - | |P34.4
| + | |
| - | |15 µl
| + | |
| - | |3 µl
| + | |
| - | |3686 bp
| + | |
| - | |951 bp
| + | |
| - | |--
| + | |
| - | | + | |
| - | | + | |
| - | |}
| + | |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker
| + | |
| - | !Sample
| + | |
| - | !Sample
| + | |
| - | !Sample
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |34.2 / 18 µl
| + | |
| - | | 34.3 / 18 µl
| + | |
| - | |34.4 / 18 µl
| + | |
| - | | + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Kleinschmidt-plasmids</u></p> | + | <ul> |
| - | <p style="font-size:15px; font-weight: bold; color: blue;"><u>Plasmid Mini-Prep</u></p>
| + | <li>plasmid: insert: pGA14_mVenus_YFP; number: P1 production date: ____ origin: ____ </li> |
| - | *experiment date: 07.06.2010 ; time: 10 – 20 h
| + | <li>plasmid: vector: pCMV_MCS; number: P2 production date: ____ origin: ____ </li> |
| - | *name of investigator: Kira, Achim, Jessy, Bea, Adrian, Hanna
| + | <li>new vector name: pCMV_mVenus_YFP <br></li> |
| - | *Kleinschmidt-plasmids
| + | <li>buffer used:3 ; Restriction-enzymes used: Enzyme XbaI (no. Lab:___) ; Enzyme PstI(no.Lab:___)</li> |
| - | <br /> | + | <li>DNA concentration (vector): 375 ng/µl ; DNA concentration (insert): 476 ng/µl</li> |
| - | <u>Glycerol Stocks</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4''' ||align="left"| '''Clone 5''' ||align="left"| '''Clone 6''' ||align="left"| '''Clone 7''' ||align="left"| '''Clone 8''' ||align="left"| '''Clone 9''' ||align="left"| '''Clone 10''' ||align="left"| '''Clone 11''' ||align="left"| '''Clone 12''' ||align="left"| '''Clone 13'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B ||align="left"| XL1B
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pXX6 ||align="left"| pKEX-2XL.Rep 40 ||align="left"| pKEX-2XL.Rep 52 ||align="left"| pKEX-2XL.Rep 68 ||align="left"| pKEX-2XL.Rep 78 ||align="left"| pCMV-VP(HS) ||align="left"| pKEX-VP1 ||align="left"| pKEX-VP2 ||align="left"| pKEX-VP3 ||align="left"| pTRUF_CMV_eGFP ||align="left"| dsAAV_CMV_eGFP ||align="left"| pTAV2||align="left"| pDG
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010 ||align="left"| 07.06.2010
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| B14 ||align="left"| B15 ||align="left"| B16 ||align="left"| B17 ||align="left"| B18 ||align="left"| B19 ||align="left"| B20 ||align="left"| B21 ||align="left"| B22 ||align="left"| B23 ||align="left"| B24 ||align="left"| B25 ||align="left"| B26
| + | |
| - | |}
| + | |
| - | | + | |
| - | <br />
| + | |
| - | <u>Given Plasmid-Number</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4''' ||align="left"| '''Clone 5''' ||align="left"| '''Clone 6''' ||align="left"| '''Clone 7'''||align="left"| '''Clone 8''' ||align="left"| '''Clone 9''' ||align="left"| '''Clone 10'''||align="left"| '''Clone 11''' ||align="left"| '''Clone 12''' ||align="left"| '''Clone 13'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| P21 ||align="left"| P22 ||align="left"| P23 ||align="left"| P24 ||align="left"| P25 ||align="left"| P26 ||align="left"| P27 ||align="left"| P28 ||align="left"| P29 ||align="left"| P30 ||align="left"| P31 ||align="left"| P32 ||align="left"| P33
| + | |
| - | |-
| + | |
| - | | align="left" | '''measured concentration''' ||align="left"|351,01 ||align="left"| 673,1 ||align="left"| 532,22 ||align="left"| 579,05 ||align="left"| 725,31 ||align="left"| 659,68||align="left"| 692,8 ||align="left"| 545,46 ||align="left"| 568,34 ||align="left"| 420,62 ||align="left"| 446,95 ||align="left"| 496,8 ||align="left"| 472,58
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <br>
| + | |
| - | '''Comments:'''
| + | |
| - | Many things went wrong today!
| + | |
| - | * Glycerol stocks must be vortexted!
| + | |
| - | * Check mini-prep buffers - especially buffer PE (needs to contain ethanol!)
| + | |
| - | * Always check volumes - try to estimate if volume makes sense (check pipettes!)
| + | |
| - | * Don't discard bacteria cultures until glycerol stocks and mini-preps are successfully done!!!
| + | |
| - | <p style="font-size:20px; font-weight: bold; color: red;">'''Today's conclusion: Better ask 2 times than do something wrong without asking!!!'''</p>
| + | |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Site-directed mutagenesis of pAAV_iGEM-MCS (PstI)</u></p>
| + | |
| - | <br>
| + | |
| - | '''Quickchange site directed mutagenesis:'''
| + | |
| - | PCR reaction:
| + | |
| - | * 2.5 µL 10x Pfu Ultra II buffer
| + | |
| - | * 0.5 µL template (therefore the a 1:20 dilution of the pAAV_iGEM-MCS (433 ng/µL) was prepared) = 10.825 ng
| + | |
| - | * 0.56 µL primer 1 (of 1:10 dilution)
| + | |
| - | * 0.56 µL primer 2 (of 1:10 dilution)
| + | |
| - | * 1 µL DMSO (primers form very strong secondary structures)
| + | |
| - | * 0.5 µL dNTP
| + | |
| - | * 18.88 µL dH<sub>2</sup>O
| + | |
| - | * 0.5 µL PfuUltra II fusion (1.25 U)
| + | |
| - | -> end volume: 25 µL
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''PCR program:'''
| + | |
| - | <br>
| + | |
| - | 1 x : 2' 95°C (HotStart polymerase)
| + | |
| - | 20 x : 30 s 95°C -> 1' 55°C -> 5' 68°C
| + | |
| - | 1 x : 4°C (over night)
| + | |
| - | <br>
| + | |
| - | Experiment will be continued tomorrow.
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ====24. Labortag 08.06.2010: Cloning of mVenus_YFP into pAAV_iGEM-MCS, continuation of site-directed mutagenesis====
| + | |
| - | | + | |
| - | Investigators: Kira, Jessy, Hanna, Achim <br>
| + | |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Analysis of iGEM-MCS sequence (RFC25 without EcoRI and NotI):</u></p> <br>
| + | |
| - | [[File:Freiburg10 iGEM-MCS.jpg|800x800px|]] <br>
| + | |
| - | The alignment with the theoretical pAAV_iGEM-MCS delivered a "C-deletion" within the NgoMIV restriction site. Due to that the pAAV_iGEM-MCS lacks this site. We controlled the bill of delivery and noted that the deletion must be GATC's fault.
| + | |
| - | All in all this doesn't matter, because parts which are in iGEM standard can be inserted and therefore they will deliver the lacking NgoMIV restriction site. Further on this could be an advantage because after digestion the fragment which is cut out can be better detected in the gel.
| + | |
| - | <br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Insertion of mVenus_YFP into pAAV_iGEM-MCS</u></p>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Digestion:</p>
| + | |
| - | | + | |
| - | <li>plasmid: insert: pGA14mVenusGeneart; number: - origin:Sven
| + | |
| - | <li>plasmid: vector: pAAV_iGEM-MCS; number: P32.2 production date:05.06.2010 origin: ____ | + | |
| - | <li>new vector name: pAAV_iGEM-MCS_mVenus <br> | + | |
| - | <li>buffer used:4 ; Restriction-enzymes used: Enzyme AgeI (no. Lab:149); Enzyme XbaI (no. Lab: 63) | + | |
| - | <li>DNA concentration (vector):433,78ng/µl ; DNA concentration (insert): 530 ng/µl<br /><br /> | + | |
| | | | |
| | {| border="1" | | {| border="1" |
| - | | components || align="right" | V (pAAV_iGEM-MCS)/ µl ||align="right"| I(pGA14mVenus_Geneart) / µl | + | | components || align="right" | V (pGA_mVenus_YFP)/ µl ||align="right"| V(pCMV_MCS) / µl |
| | |- | | |- |
| - | | DNA || align="right" | 2,3||align="right"|3,8 | + | | DNA || align="right" | 4||align="right"|2,7 |
| | |- | | |- |
| | | BSA (10x) || align="right" |2||align="right"|2 | | | BSA (10x) || align="right" |2||align="right"|2 |
| Line 810: |
Line 260: |
| | | Buffer 3 (10x)|| align="right" |2||align="right"|2 | | | Buffer 3 (10x)|| align="right" |2||align="right"|2 |
| | |- | | |- |
| - | |Enzyme: AgeI (no.Lab:149)|| align="right" |1,25||align="right"|1,25 | + | |Enzyme: XbaI (no.Lab:___)|| align="right" |1,5||align="right"|1,5 |
| | |- | | |- |
| - | |Enzyme: XbaI (no.Lab:63)|| align="right" |0,75||align="right"|0,75 | + | |Enzyme: PstI (no.Lab:___)|| align="right" |1||align="right"|1 |
| | |- | | |- |
| - | |H<sub>2</sup>O|| align="right" |11,7||align="right"|10,2 | + | |H2O|| align="right" |9,5||align="right"|10,8 |
| | |- | | |- |
| | |'''Total volume'''|| align="right" |<b>20</b>||align="right"|<b>20</b> | | |'''Total volume'''|| align="right" |<b>20</b>||align="right"|<b>20</b> |
| | |} | | |} |
| | | | |
| - | <li> Incubation: 1 1/2 h at 37°C<br> | + | <li> Incubation: 1 h at 37°C</li> |
| | + | </ul> |
| | | | |
| - | 0.5 g Agarose, 50 ml TAE (1%), 3 µL GELRED (3-6µl), at 115 Volt, running time:
| + | <b>1% Agarose gel and Gel extraction</b> |
| - | <br /> | + | <ul> |
| - | <br /> | + | <li>prepare 1% agarose gel, run gel for 45 minutes(119 V)</li> |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | <li>cut out insert and vector</li> |
| - | !Sample
| + | <li>perform gel extraction following standard protocol provided by Qiagen</li> |
| - | !Sample/µl]
| + | </ul> |
| - | !Loading dye (6x)/µl
| + | |
| - | !Expected size 1 (Geneious)
| + | |
| - | !Expected size 2 (Geneious)
| + | |
| - | |--
| + | |
| - | |P34.2
| + | |
| - | |20 µl
| + | |
| - | |4 µl
| + | |
| - | |4617 bp
| + | |
| - | |22 bp
| + | |
| | | | |
| - | |--
| + | <b>Ligation</b> |
| - | |pGA14mVenus
| + | |
| - | |20 µl
| + | |
| - | |4 µl
| + | |
| - | |2870 bp
| + | |
| - | |774 bp
| + | |
| - | |}
| + | |
| - | <br /><br /> | + | |
| - | <li>Gel loaded with vector and insert samples, 24 µl each & 8 µl marker
| + | |
| - | <li>after 20 minutes, the insert band was cut out. Two overlapping bands were visible in the vector well, after 1 1/2 hours those bands were separated and both were cut out. <br /><br />
| + | |
| | | | |
| - |
| |
| - | {| border="1"
| |
| - | | Sample|| align="right" | weight || align="right" | concentration
| |
| - | |-
| |
| - | | Vektor_Oben || align="right" |0,32 g || align="right" |29,8 ng/µl
| |
| - | |-
| |
| - | | Vektor_Unten || align="right" |0,14 g || align="right" |12,8 ng/µl
| |
| - | |-
| |
| - | | Insert|| align="right" |0,3 g || align="right" |12,9 ng/µl
| |
| - |
| |
| - | |}
| |
| - | <br /><br />
| |
| - | <li>the exact volume of insert and vector was calculated with LabTools:
| |
| - | <li>Ligation I with Vektor_Oben:
| |
| | <ul> | | <ul> |
| - | *Vector: 4,16 µl
| + | <li>Measure DNA-concentration with Nanodrop </li> |
| - | *Insert: 4,84 µl
| + | <li>c(mVenus_YFP) = 16,8 ng/µL</li> |
| | + | <li>c(pCMV_MCS) = 22,8 ng/µL</li> |
| | + | <li>Calculation of volume needed for ligation: |
| | + | <li>c(mVenus_YFP) = 3,66 µL</li> |
| | + | <li>c(pCMV_MCS) = 5,34 µL</li></li> |
| | </ul> | | </ul> |
| - | <li>Ligation II with Vektor_Unten: | + | |
| | + | <b>Transformation</b> |
| | <ul> | | <ul> |
| - | *Vector: 6 µl
| + | <li>Transformation has been followed the standard protocol [[Media:Freiburg10_Cloning Protocol.pdf]] </li> |
| - | *Insert: 3 µl
| + | |
| | </ul> | | </ul> |
| | | | |
| | + | ===12. Labortag 20.05.2010=== |
| | | | |
| | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Picking clones</b></p>==== |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>continuation of site-directed mutagenesis</u></p> | + | <p><b>Investigators: Adrian, Bea</b></p> |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Digestion with DpnI:</p> | + | <ul><br/> |
| - | <li>0.5 µl DpnI added
| + | Clones were picked according to the standard protocol. |
| - | <li>incubated for 1 hour at 37°C | + | *3 approaches from each plate</li> |
| - | | + | </ul> |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Trafo:</p>
| + | |
| | | | |
| - | <li>Cells used for transformation: XL1B
| + | ====13. Labortag 21.05.2010: CMV-Promoter==== |
| - | <li>Centrifugation for 3 min at 8000 rpm instead of 6000 rpm (accidentaly)
| + | |
| - | <li>Plated on agar plate: pAAV_IGEM-MCS Mutagenese PST1 8.6.2010 AM XL1B
| + | |
| - | <li>incubated over night
| + | |
| | <br> | | <br> |
| | + | <b>Theoretical cloning:</b> (Volker, Hanna) |
| | + | * The CMV promoter (mainly the regulatory region) was further characterized: For this purpose U.S. patent no. 5,385,839 was used. [[Media:Freiburg10_Patent_US5385839A.pdf]] |
| | + | * Further on the CMV promoter sequence was blasted. The results delivered a 98% query coverage with the "Human herpesvirus 5 strain Toledo, complete genome" (accession no.: GU937742.1). Interestingly the maximal identity was just 99%. This can be explained due to a nucleotide deletion and a C-T transition (red circles), which were also marked in Geneious. |
| | + | [[File:Freiburg10_CMV-Alignment.jpg|500x500px|]] |
| | <br> | | <br> |
| - | ====25. Labortag 09.06.2010: pAAV_iGEM-MCS_mVenus-YFP, site-directed mutagenesis====
| + | [[File:Freiburg10 CMV.jpg|800x800px|]] |
| - | investigators: Jessy, Achim, Sven, Toby, Hanna
| + | |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: red;">'''No pAAV_iGEM-MCS_w/oPstI transformed bacteria (site-directed mutagensis) grew on the ampicillin agar plates over night! '''</p> Experiment is conducted again by Toby (Thanks a lot!!!).<br>
| + | '''To do''': find "+1"-location (transcription start); which transcription factors bind to the regulatory region of the CMV promoter? |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: red;">'''Further on the cloning of mVenus-YFP into pAAV_iGEM-MCS seemed to fail: '''</p> Only on the agar plate cotaining the bacteria transformed with the "vector_oben" ligation, which actually should be the "wrong" ligation (gelextraction of a band which contained fragments that were too large, see picture in lab journal), revealed colonies. Therefore this experiments is also conducted one more time by Sven (thanks also a lot!!!)<br>
| |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Insertion of mVenus_YFP into pAAV_iGEM-MCS</u></p>
| + | '''LB medium''' was prepared: (Patrick and Chris W.) |
| - | *experiment date: 9.6.2010
| + | * 10 g Bacto-Tryptone, 5 g Bacto-Yeast, 10 g NaCl were mixed in 500 mL milipore-H2O. |
| - | *name of investigator: Sven | + | * Volume was adjusted to 1 L with milipore-H2O. |
| - | *plasmid:
| + | * 100 mL flasks were each filled with 50 mL medium. |
| - | **Vector: name: pAAV_iGEM-MCS number: 34.2 production date: 5.6.2010
| + | * 0.75 g agar was added to each flask. |
| - | **Insert: name: pOG14_mVenus number: - production date: ____ origin: Sven :) | + | * LB was sterilized by autoclaving and is now stored at room temperature. |
| - | *new vector name: pAAV_iGEM-MCS_mVenus-YFP | + | <br> |
| - | *buffer used: NEB4 ; Restriction-enzymes used: Enzyme 1 (no. Lab:___) AgeI ; Enzyme 2 (no.Lab:___) XbaI | + | <br> |
| - | *DNA concentration (vector): 433 ng/µL ; DNA concentration (insert): 530 ng/µL | + | <b>Plasmid Mini-Prep according to the standard protocol</b> |
| - | <br /> | + | |
| - | <br /> | + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Digestion</p> | + | |
| - | {| border="1"
| + | |
| - | | components || align="right" |volume of vector /µl || align="right" |volume of insert /µl
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |2.83 || align="right" | 2.77
| + | |
| - | |-
| + | |
| - | | BSA (100x) || align="right" | 0.5 || align="right" | 0.5
| + | |
| - | |-
| + | |
| - | | Buffer NEB4 (10x)|| align="right" | 3.0 || align="right" | 3.0
| + | |
| - | |-
| + | |
| - | |Enzyme 1 (AgeI)|| align="right" | 1.75 || align="right" | 1.75
| + | |
| - | |-
| + | |
| - | |Enzyme 2 (XbaI)|| align="right" | 1.25 || align="right" | 1.25
| + | |
| - | |-
| + | |
| - | |H<sub>2</sup>O|| align="right" |20.73 || align="right" | 20.67
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 30 || align="right" | 30
| + | |
| - | |}
| + | |
| | | | |
| - | <br /><br /> | + | <ul> |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Agarose-Gel:</p> | + | <li>investigator: Adrian, Kira, Anna, Chris W., Patrick</li> |
| - | <br /> | + | <li>Measure DNA-concentration with Nanodrop</li> |
| - | 0.5 g Agarose, 50 ml TAE (1 %), 3 µL GELRED (3-6µl), at 115 Volt
| + | </ul> |
| - | <br /> | + | |
| - | <br /> | + | |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Gelextraction</p>
| |
| - | <br />
| |
| - | * insert (mVenus-YFP): 26 ng/µL
| |
| - | * vector (pAAV_iGEM-MCS): 30 ng/µL
| |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Ligation</p>
| |
| - | <br />
| |
| - | * vector: 5.85 µL
| |
| - | * insert: 3.15 µL
| |
| - | * ligase: 1 µL
| |
| - | * buffer: 10 µL
| |
| - | <br>
| |
| - | <br />
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Test digestion of pAAV_iGEM-MCS</u></p>
| |
| - | p style="font-size:15px; font-weight: bold; color: blue;">Test digestion</p>
| |
| - | *buffer used: 1 ; Restriction-enzymes used: Enzyme 1 (no. Lab:___) NgoMIV
| |
| - | *Plasmid
| |
| - | **Given Plasmid-Number: P34.2 ; DNA concentration: 433.78 ng/µL;
| |
| - | **Given Plasmid-Number: P34.3 ; DNA concentration: 408.48 ng/µL;
| |
| - | **Given Plasmid-Number: P34.4 ; DNA concentration: 409.8 ng/µL;
| |
| - | <br />
| |
| - | <br />
| |
| | {| border="1" | | {| border="1" |
| - | | align="left" | '''Components''' ||align="left"| '''P34.2 [µL] ''' ||align="left"| '''P34.3 [µL] '''||align="left"| '''P34.4 [µL] ''' | + | | || align="right" | P3 (pCMV_mVenus_YFP) ||align="right"| P4 (pCMV_mVenus_YFP) ||align="right"| P5 (pCMV_mVenus_YFP) |
| | |- | | |- |
| - | | align="left" | DNA ||align="left"| 2.3 ||align="left"| 2.5 ||align="left"| 2.4 | + | | concentration (ng/µl)|| align="right" | 457||align="right"|470,8|| align="right" | 477,29 |
| - | |-
| + | |
| - | | align="left" | BSA (10x) ||align="left"| 1 ||align="left"| 1 ||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | Buffer no. 1 (10x) ||align="left"| 1 ||align="left"| 1 ||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 1 (no. Lab: 113) ngoMIV ||align="left"| 1 ||align="left"| 1 ||align="left"| 1
| + | |
| - | |-
| + | |
| - | |-
| + | |
| - | | align="left" | H<sub>2</sup>O ||align="left"| 4.7 ||align="left"| 4.5 ||align="left"| 4.6
| + | |
| - | |-
| + | |
| - | | align="left" | '''Total volume''' ||align="left"| '''10''' ||align="left"| '''10''' ||align="left"| '''10'''
| + | |
| | |} | | |} |
| | | | |
| - | *Incubation: 1 h, 37°C
| + | ===14. Labortag 25.05.2010=== |
| - | <br />
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Agarose-Gel:</p>
| + | |
| - | <br />
| + | |
| - | 0.5 g Agarose, 50 ml TAE (1 %), 3 µL GELRED, at 115 Volt, running time: 45 minutes + 20 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample/µl]
| + | |
| - | !Loading dye (6x)/µl
| + | |
| - | !Expected size 1 (Geneious)
| + | |
| - | !Expected size 2 (Geneious)
| + | |
| - | |--
| + | |
| - | |P34.2
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |3728 bp
| + | |
| - | |903 bp
| + | |
| - | |--
| + | |
| - | |P34.3
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |3782 bp
| + | |
| - | |903 bp
| + | |
| - | |--
| + | |
| - | |P34.4
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |3782 bp
| + | |
| - | |903 bp
| + | |
| - | |--
| + | |
| | | | |
| - | |}
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Cloning of pCMV_mVenus_YFP + pAAV_MCS</b></p>==== |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker
| + | |
| - | !Sample P34.2 /µl
| + | |
| - | !Sample P34.3 /µl
| + | |
| - | !Sample P34.4 /µl
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | | 8 µL
| + | |
| - | | 10 µL
| + | |
| - | | 10 µL
| + | |
| - | | 10 µL
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| - | '''Comments:''' Digestion of P34.2 delivered just one fragment size, revealing that - as expected after sequencing (C-deletion: no NgoMIV site in iGEM-MCS) - there was just one NgoMIV restriction site in the vector. In contrast to that the digestion of P34.3 and P34.4 delivered two bands - whereas the band of the smaller fragments looked like a smier. Because of this obscure and non-satisfying results (besides our assumption that there's something wrong with the marker :) )we decided to sequence the P34.3 vector:
| + | |
| - | * c(P34.3): 408.48 ng/µL
| + | |
| - | * Volume (plasmid): 5.14 µL
| + | |
| - | * Volume (vector): 24.86 µL
| + | |
| - | * Name of eppi: HW_34
| + | |
| - | * Primer: GATC_std_pTeSp-1
| + | |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Picking of Clones from pAAV_iGEM-MCS_mVenus-YFP_Vektor_oben</u></p> | + | <p><b>Investigators: Kira, Anna, Volker, Jessica</b></p> |
| - | *experiment date: 9.6.2010
| + | |
| - | *name of investigator: Hanna, Achim
| + | |
| - | *plasmid:
| + | |
| - | **Vector: name: pAAV_iGEM-MCS_Vektor_oben
| + | |
| - | **Insert: name: pOG14_mVenus
| + | |
| - | *new vector name: pAAV_iGEM-MCS_mVenus-YFP_Vektor_oben
| + | |
| - | <br />
| + | |
| - | The Clones were picked and incubated in 10 mL LB-Medium containing 10µL ampicillin over night at 37°C on a rotary shaker.
| + | |
| | | | |
| - | ===26. Labortag 10.06.2010:===
| |
| - | ====Site-directed mutagenesis of PstI in ITRs====
| |
| - | <p style="font-size:12px; font-weight: bold; color: blue;">1. Site directed mutagenisis</p><br>
| |
| - |
| |
| - | * Aim: deletion of PstI from both ITR's
| |
| - | * 2 PCR tubes got prepared, one for the left ITR and one for the right. (The SDM was performed according to the standart protocol)
| |
| - | * [[Media:Freiburg10_Site_directed_Mutagenesis_pAAV_MCS_deletion_PstI.pdf]]
| |
| | <br> | | <br> |
| - | ====Mini-Prep of pAAV_iGEM_Venus_YFP and test digestion====
| |
| - | <p style="font-size:12px; font-weight: bold; color: blue;">2.1 Mini-Prep of pAAV_iGEM_mVenus_YFP</p><br>
| |
| | | | |
| - | Title: '''pAAV_iGEM_mVenus_''' <br>
| + | <li>plasmid: insert: pCMV_mVenus_YFP; number: P5 production date: 21.05.2010 origin: ____ |
| - | investigator: Jessy, Achim <br>
| + | <li>plasmid: vector: pAAV_MCS; number: P? production date: ____ origin: ____ |
| - | <u>Glycerol Stocks</u>
| + | <li>new vector name: pAAV_mVenus_YFP <br> |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| XL1 blue ||align="left"| XL1 blue
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pAAV_iGEM_mVenus||align="left"| pAAV_iGEM_mVenus
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 10.06.2010 ||align="left"| 10.06.2010
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| B30 ||align="left"| B31
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| - | <u>Given Plasmid-Number</u> | + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''given number''' ||align="left"| P35 ||align="left"| P36
| + | |
| - | |}
| + | |
| - | <br /> | + | |
| | | | |
| - | Title: '''pAAV_iGEM_mVenus_YFP''' (guided by Sven) <br>
| |
| - | investigator: Bea
| |
| - | <u>Glycerol Stocks</u>
| |
| - | {| border="1"
| |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4'''
| |
| - | |-
| |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21 ||align="left"| BL-21 ||align="left"| BL-21
| |
| - | |-
| |
| - | | align="left" | '''Plasmidname''' ||align="left"| pAAV_iGEM_mVenus_YFP ||align="left"| pAAV_iGEM_mVenus_YFP ||align="left"| pAAV_iGEM_mVenus_YFP ||align="left"| pAAV_iGEM_mVenus_YFP
| |
| - | |-
| |
| - | | align="left" | '''Date''' ||align="left"| 10.06.2010 ||align="left"| 10.06.2010 ||align="left"| 10.06.2010 ||align="left"| 10.06.2010
| |
| - | |-
| |
| - | | align="left" | '''given number''' ||align="left"| B32 ||align="left"| B33 ||align="left"| B34 ||align="left"| B35
| |
| - | |}
| |
| - | <br />
| |
| - | <u>Given Plasmid-Number</u>
| |
| - | {| border="1"
| |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' ||align="left"| '''Clone 4'''
| |
| - | |-
| |
| - | | align="left" | '''given number''' ||align="left"| P37 ||align="left"| P38 ||align="left"| P39 ||align="left"| P40
| |
| - | |}
| |
| - | <br />
| |
| - | <p style="font-size:12px; font-weight: bold; color: blue;">2.2 Test digestion</p>
| |
| - | *buffer used: 4 ; Restriction-enzymes used: Enzyme XbaI (no. Lab:___) ; Enzyme AgeI (no.Lab:___) ____
| |
| - | *Plasmid
| |
| - | **Given Plasmid-Number: P37; DNA concentration: 114,8 ng/µL ;
| |
| - | **Given Plasmid-Number: P38 ; DNA concentration: 179,8 ng/µL;
| |
| - | **Given Plasmid-Number: P39 ; DNA concentration: 180,8ng/µL ;
| |
| - | **Given Plasmid-Number: P40; DNA concentration: 189,2 ng/µL;
| |
| - | <br />
| |
| - | '''Comments:''' Clones were picked from trafo-plate in the morning and mini-prep was performed in the late afternoon. Due to this and the fact that bacterial strain was BL-21, DNA-concentrations are quite low.
| |
| | <br> | | <br> |
| - | <br> | + | '''1st try: |
| - | Investigators: Bea, Chris W. <br>
| + | |
| | + | <li>buffer used:3 ; Restriction-enzymes used: Enzyme NotI (no. Lab:46) |
| | + | <li>DNA concentration (vector): 360 ng/µl ; DNA concentration (insert): 477 ng/µl |
| | | | |
| | {| border="1" | | {| border="1" |
| - | | align="left" | '''Components''' ||align="left"| '''Volume/µL''' ||align="left"| '''Mastermix''' ||align="left"| '''Sample: P37''' ||align="left"| '''Sample: P38''' ||align="left"| '''Sample: P39''' ||align="left"| '''Sample: P40''' | + | | components || align="right" | V (pCMV_mVenus_YFP)/ µl ||align="right"| V(pAAV_MCS) / µl |
| | |- | | |- |
| - | | align="left" | DNA ||align="left"| 800 ng ||align="left"| - ||align="left"|7,0 µL||align="left"|4,5 µL||align="left"|4,4 µL||align="left"| 4,2 µL
| + | | DNA || align="right" | 4.2||align="right"|3.5 |
| | |- | | |- |
| - | | align="left" | BSA (10x) yes ||align="left"| 1,5 µL ||align="left"| 7,5 µL ||align="left"|MM 4,5 µL||align="left"|MM 4,5 µL||align="left"|MM 4,5 µL||align="left"| MM 4,5 µL
| + | | BSA (10x) || align="right" |3||align="right"|3 |
| | |- | | |- |
| - | | align="left" | Buffer no. 4 (10x) ||align="left"| 1.5 µL||align="left"| 7,5 µL ||align="left"|"||align="left"|"||align="left"|"||align="left"| "
| + | | Buffer 3 (10x)|| align="right" |3||align="right"|3 |
| | |- | | |- |
| - | | align="left" | Enzyme 1 (no. Lab: ) AgeI ||align="left"| 0.75 µL ||align="left"| 3,75 µL ||align="left"|"||align="left"|"||align="left"|"||align="left"| "
| + | |Enzyme: NotI (no.Lab:46)|| align="right" |1||align="right"|1 |
| | |- | | |- |
| - | | align="left" | Enzyme 2 (no. Lab: ) XbaI ||align="left"| 0.5 µL||align="left"| 2,5 µL ||align="left"|"||align="left"|"||align="left"|"||align="left"| " | + | |H2O|| align="right" |15.3||align="right"|15.3 |
| | |- | | |- |
| - | | align="left" | H<sub>2</sup>O ||align="left"| variable ||align="left"| - ||align="left"|3,75 µL||align="left"|0,25 µL||align="left"|6,35 µL||align="left"| 6,55 µL
| + | |'''Total volume'''|| align="right" |<b>26.4</b>||align="right"|<b>25.8</b> |
| - | |-
| + | |
| - | | align="left" | '''Total volume''' ||align="left"| '''15 µL''' ||align="left"| - ||align="left"|15 µL||align="left"|15 µL||align="left"|15 µL||align="left"| 15 µL
| + | |
| | |} | | |} |
| - | <br />
| |
| | | | |
| - | *Incubation: 45 min, 37°C
| + | <li> Incubation: 1 1/2 h at 37°C |
| - | <br /> | + | |
| - | | + | |
| - | ===27. Labortag 11.06.2010:===
| + | |
| - | in additon to<p style="font-size:12px; font-weight: bold; color: blue;">Site directed mutagenisis</p> on 10.06.10
| + | |
| - | | + | |
| - | | + | |
| - | '''transformation'''<br>
| + | |
| - | transformation was performed according to the standard protocol [[Media:Freiburg10_Cloning Protocol.pdf]]<br>
| + | |
| - | Cells were plated on agar plates (2x 1:100; 2x pellet)containing ampicillin and stored over night at 37°C.
| + | |
| - | | + | |
| - | <p style="font-size:12px; font-weight: bold; color: blue;">Sequenzing</p>
| + | |
| - | p39 was send to GATC for sequenzing
| + | |
| - | | + | |
| - | * p39: 180,83 ng*µl^-1
| + | |
| - | * volume plasmid: 8,3 µl
| + | |
| - | * volume water: 21,7 µl
| + | |
| - | * primer: GATC_std_pTeSp-1
| + | |
| - | | + | |
| - | ===28. Labortag 12.06.2010:===
| + | |
| - | Kira <br>
| + | |
| - | The plates were stored in the iGEM shelf @ 4 °C. Even if the transformation was not very successfull, some colonies can be picked and inoculated either tomorrow or on Monday.
| + | |
| - | '''Note: Site-directed mutagenesis does not generate lots of intact plasmids, so few colonies are normal!! (Tobias)'''
| + | |
| | <br> | | <br> |
| - | | + | '''note: too little water was added |
| - | ===29. Labortag 13.06.2010:===
| + | |
| - | Jessy, Patrick, Hanna <br>
| + | |
| - | We weren't able to detect any colonies on the plates - just a few air bubbles :) To be entirely sure, we put them back into the 37°C room over night.
| + | |
| | <br> | | <br> |
| - |
| |
| - | ===30. Labortag 14.06.2010:===
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>pAAV_iGEM-mVenus-YFP:</u></p>
| |
| - | The sequencing data of pAAV_iGEM_mVenus-YFP was analyzed. The alignment with the "theoretical" pAAV_iGEM_mVenus-YFP showed that we successfully inserted mVenus-YFP into pAAV_iGEM-MCS.
| |
| - | [[File:Freiburg10 mVenus-Alignment.jpg|800x800px|]]
| |
| | <br> | | <br> |
| - | | + | 1% Agarose gel |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Site-directed mutagenesis:</u></p>
| + | |
| - | Also today no colonies were detectable on any plates!
| + | |
| | <br> | | <br> |
| - | | + | <li>1% agarose gel was prepared, gel ran for 45 minutes(119 V) |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>TK-GMK-Plasmid</u></p> | + | |
| - | Adrian, Hanna <br>
| + | |
| - | puB6_V5_His6_clone1 + TK/GMK (P15) will be sequenced again: <br>
| + | |
| - | * name: AF 1
| + | |
| - | * primer: GATC_std_BGH-reverse
| + | |
| - | * volume(plasmid) = 4.25 µL
| + | |
| - | * volume (H<sub>2</sup>O) = 25.75 µL
| + | |
| - | Results (15.06.): Unfortunately just ~ 300 bp were sequenced. <br>
| + | |
| - | <p style="font-size:13px; font-weight: bold; color: purple;">To do: Therefore more primers have to be designed in order to sequence the whole ~2000 bp TK-GMK fusion construct! </p>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Zellkultur</u></p>
| + | |
| - | AAV 293 and HT1080 Cells have been splitted and plated out on 10 cm dishes for transfektion.
| + | |
| - | The Cells were accounted by using the Neubauer-Meteringchamber.
| + | |
| - | After harvesting by following the standard protocol the pallet have been resuspendiated in 15ml of DTT medium.
| + | |
| - | 2,5µl of this cell suspension have been mixed with 47.5µl of trypan blue.
| + | |
| - | | + | |
| - | We counted 12,5x 10^6 cells/ml for the T293 AAV cell line and 10x10^6 cells /ml for the HT1080 cell line.
| + | |
| - | | + | |
| - | The AAV 293 cells have been plated out on four dishes with 250µl of the cell suspension, on one plated with 1ml and on another dish
| + | |
| - | with 1.5ml.
| + | |
| - | note that that the date is wrong on the plates and the flasks! Cells have been already plated out on the 14th. of June.
| + | |
| - | | + | |
| - | We also seated two flasks one for each cell line. Therefor we used 1ml of the HT1080 cell suspension and two ml of the the AAV293 cells.
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| | + | '''2nd try:''' |
| | | | |
| - | ===31. Labortag 15.06.2010:===
| + | <li>buffer used:4 ; Restriction-enzymes used: Enzyme NotI (no. Lab:159) |
| - | Adrian, Achim, Chris W., Hanna
| + | <li>DNA concentration (vector): 360 ng/µl ; DNA concentration (insert): 477 ng/µl |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Solutions for transfection were prepared:</u></p>
| + | |
| - | * 1 x TE-Buffer: 1.2114 g TRIS, 0.2 mL EDTA, 81 mL milipore-H<sub>2</sup>O were mixed. pH was adjusted with HCl (1M and 5M) to 7.50. Volume was adjusted to 100 mL with milipore-H<sub>2</sup>O. Solution was autoclaved (sterilization by filtration is also possible). <br>
| + | |
| - | * 1 M CaCl2: 147.02 g CaCl2 x 2H<sub>2</sup>O (M = 147.02 g/mol) was solved in 1 Liter H<sub>2</sup>O and sterilized by filtration.<br>
| + | |
| - | * 2 x HBS: 0.027 g Na2HPO4, 1.636 g NaCl, 1.19155 g HEPES were dissolved in ~ 30 mL milipore-H<sub>2</sup>O. pH was adjusted to 7.10. Volume was adjusted to 100 mL and sterilized by filtration.<br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ===32. Labortag 16.06.2010:===
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Sponsoring:</u></p>
| + | |
| - | Anna, Kerstin, Anissa<br>
| + | |
| - | Sponsoring letters were examined and modified one more time. <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Sequencing of TK-GMK:</u></p>
| + | |
| - | Hanna<br>
| + | |
| - | Two primers were designed and ordered from Sigma-Aldrich. Probably we will receive them on Friday.<br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Mega primer PCR:</u></p>
| + | |
| - | Bea, Hanna <br>
| + | |
| - | <br>
| + | |
| - | [[File:Freiburg10 ITRprimer.jpg]]
| + | |
| - | | + | |
| - | ===33. Labortag 17.06.2010:===
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: #014421;"><u>Primer designing: VP1 primer for pKEX forward/reverse </u></p>
| + | |
| - | Investigator: Volker<br>
| + | |
| - | The construnts that we receieved from PD Dr. Kleinschmidt (DKFZ) are mainly in the pKEX backbone. The sequences of the backbone and the inserts are not availible, for this reason we decided to sequence the constructs. The first step is to sequence from one construct into the backbone. In the second step we will designe primers that bind in the backbone and point into the direction of the insert.
| + | |
| - | These primers will then be used to sequence the inserts of all the constructs.<br>
| + | |
| - | | + | |
| - | '''Forward primer pKEX in VP1 from bp 4124 to 414'''
| + | |
| - | *VP1 primer for pKEX forward: 5' - CAACAAGTCTGTTAATGTGGAC - 3' 22bp; TM: 50°C; CG% 41%
| + | |
| - | | + | |
| - | '''Reverse primer pKEX in VP1 from bp 2081 to 2100'''
| + | |
| - | *VP1 primer for pKEX reverse: 5' - GTGGGCCAGGTTTGAGCTTC - 3' 20bp; TM: 54°C CG%: 60%
| + | |
| - | | + | |
| - | The amplicon that should be produced by the primers is 199 bp long.
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: #014421;"><u>Primer designing: CMV_forward/reverse_qPCR </u></p>
| + | |
| - | Investigator: Volker<br>
| + | |
| - | Primers for the titering of the CMV region from the literature [Rohr et al., 2002] and [Rohr et al., 2005] were compared and analysed.
| + | |
| - | The sequences from 2002 showed a differce in one nucleotide compared to the other primer and to our sequence. For this reason the second set [Rohr et al., 2005] was ordered.<br>
| + | |
| - | | + | |
| - | *CMV_forward_qPCR: 5' - GGGACTTTCCTACTTGGCA - 3'
| + | |
| - | *CMV_reverse_qPCR: 5' - GGCGGAGTTGTTACGACA - 3'
| + | |
| - | | + | |
| - | [[File:Freiburg10_Binding of the CMV_qPCR primer in the vector plasmid.jpg|900px|left|thumb|Obtaining fragments by digestion with AlwNI. Fragments contain left and right ITR respectively]]<br>
| + | |
| - | | + | |
| - | <p style="clear:both;">These primers will be used in a quantitative PCR assay with Sybr-green to measure the genomic titer and the infectious titer of the viral particles we will produce in the future.</p>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: #014421;"><u>Ordering of required reagents: </u></p>
| + | |
| - | Investigator: Volker<br>
| + | |
| - | | + | |
| - | *Nuclease S1 was ordered from Promega (10000Units for 36,00€)
| + | |
| - | *Proteinase K was ordered from Sigma (5mg for 31,30€; BioUltra, ≥30 units/mg protein, lyophilized powder)
| + | |
| - | These reagents are required for the procedure to determine the genomic and the infectious titer.<br><br>
| + | |
| - | | + | |
| - | ===34. Labortag 18.06.2010: Transfection===
| + | |
| - | ====Transfection====
| + | |
| - | Transfection via calcium phosphat with the prepared solutions (31. Labortag 15.06.2010)
| + | |
| - | <br>
| + | |
| - | | + | |
| - | '''investigators:''' Chris W., Hanna, Patrick, Adrian, Volker<br>
| + | |
| - | Transfections with the following plasmids:
| + | |
| - | | + | |
| - | 1)
| + | |
| - | * pAAV_iGEM_mVenus_YFP (glycerol stock B34, P39) P39 has the correct sequence (confirmed)
| + | |
| - | * pHelper
| + | |
| - | * pAAV_RC
| + | |
| - | 2)
| + | |
| - | * pAAV_iGEM_mVenus_YFP (glycerol stock B33, P38) P38 (we dont know if P39 has the correct sequence!)was used because the amount of P39 ''[[was not sufficient enough]]'' for four Transfections!
| + | |
| - | * pHelper
| + | |
| - | * pAAV_RC
| + | |
| - | | + | |
| - | Plasmid concentrations: <br>
| + | |
| - | pAAV_RC: 1 µg/µl <br>
| + | |
| - | pHelper: 280 ng/µl <br>
| + | |
| - | pAAV_iGEM_mVenus_YFP, P39: 180,83 ng/µl <br>
| + | |
| - | pAAV_iGEM_mVenus_YFP, P38: 179,75 ng/µl <br>
| + | |
| - | | + | |
| - | Cellculture clone 1 and 2 were transfected with P39. Cellculture clone 3 and 4 were transfected with P38. <br>
| + | |
| - | | + | |
| - | Deviations from the standard protocol (currently incomplete):
| + | |
| - | We could only pipet 3,3 µg (instead of 10 µg) from each of the 3 plasmids into the 15 ml falcons due to insufficient amount of plasmid.
| + | |
| - | | + | |
| - | Adrian tried to examine the precipation of the CaCl2+ DNA Clusters. We have to optimize the pH of our 2xHBS at the moment it is 11.12
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ====Theoretical: Digestion of pAAV_MCS for PCR to design ITR BioBrick====
| + | |
| - | '''investigator''': Bea <br />
| + | |
| - | '''idea:'''
| + | |
| - | <ul>
| + | |
| - | <li>Digest vector in order to obtain two fragments which contain the left and the right ITR respectively. </li> | + | |
| - | <li>Separation (Agarose-gel) and gel extraction of fragments</li>
| + | |
| - | <li>Perform PCR with iGEM-Primers (forward primer contains EcoRI and Xbai; reverse primer contains SpeI and PstI)</li>
| + | |
| - | <li>to do:</li>
| + | |
| - | * design primers
| + | |
| - | * digestion of pAAV_MCS etc.
| + | |
| - | </ul>
| + | |
| - | '''Theoretical:''' Digestion of pAAV_MCS with AlwNI produces two fragments which can be separated by agaorse gel.
| + | |
| - | <br>
| + | |
| - | [[File:Freiburg10 pAAV MCS fragments cut with AlwNI preparation for PCR.jpg|900px|thumb|left|Obtaining fragments by digestion with AlwNI. Fragments contain left and right ITR respectively]]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ===35. Labortag 19.06.2010: Literature seminar===
| + | |
| - | | + | |
| - | '''Investigators: Bea, Hanna, Melanie, Denis, Volker, Christian W., Adrian and Achim'''
| + | |
| - | | + | |
| - | Topics:
| + | |
| - | *The ITRs
| + | |
| - | *The Cap
| + | |
| - | | + | |
| - | ===36.Labortag 20.06.2010: Picking clones of sequenced pAAV_igEM_mVenus_YFP===
| + | |
| - | '''Investigators: Bea and Chris W'''
| + | |
| - | <ul>
| + | |
| - | <li>Incoluate 250 mL DYT medium (add 250µL ampiciliin) with B34 (glycerol stock: BL-21 E.coli cells with P39 plasmid: pAAV-iGEM_mVenus_YFP </li>
| + | |
| - | <li>Incubate on rotary shaker over-night (o/n) at 37°C </li>
| + | |
| - | <li>to do: perform Midi-Prep tomorrow (21.06.2010)</li>
| + | |
| - | </ul>
| + | |
| - | | + | |
| - | ===37.Labortag 21.06.2010: Second Transfection, Transduction===
| + | |
| - | | + | |
| - | 2x HBS was steril filtrated into two 50 ml flacons. The falcons were put into the cellculture fridge.
| + | |
| - | | + | |
| - | New medium is prepared:
| + | |
| - | | + | |
| - | DMEM-Medium
| + | |
| - | *500ml DMEM with Glutamax
| + | |
| - | *10% FCS (50 ml)
| + | |
| - | *5 ml 100x Pen/Strep
| + | |
| - | *5 ml 100x NaPyruvat
| + | |
| - | | + | |
| - | H-DMEM-Medium
| + | |
| - | *400 ml DMEM with Glutamax
| + | |
| - | *90 ml FCS
| + | |
| - | *5 ml 100x Pen/Strep
| + | |
| - | *5 ml 100x NaPyruvat
| + | |
| - | | + | |
| - | Investigators: Patrick, Johannes
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Feching dry ice for the experiments </u></p>
| + | |
| - | Volker
| + | |
| - | | + | |
| - | Dry ice can be fetched from the department for macromolecular chemistry. The chemical depot is located in the second basement. The dry ice can be retrieved from monday to friday from 11:00 to 11:30 and from 14:15 to 14:30. The requestforms for this institute can be found in the iGEM folder and should be filled out, signed by an advisor and taken to the chemical depot. A styrofoam container is required for the transport.<br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Annotation of the BRs and the PLA2 domains </u></p>
| + | |
| - | Volker<br>
| + | |
| - | The Bacis Regions which are important for the nuclear localisation were annotated in the protein sequences of VP 1-3 according to:
| + | |
| - | [[Media:Freiburg10_Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly.pdf|[Grieger et al, 2006]]]<br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Endotoxin-free Midi-Prep of pAAV_iGEM_mVenus-YFP </u></p>
| + | |
| - | Chris W., Hanna (guided by Sven) <br>
| + | |
| - | Midi-Prep was performed following standard protocol: [[File:Freiburg10 Endotoxinfreie Midi.pdf]] <br>
| + | |
| - | DNA concenration was measured: 507 ng/µL <br>
| + | |
| - | Plasmid was further used for a... <br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>... Second Transfection</u></p>
| + | |
| - | Six transfections were performed according to the standart protocol: [[Media:Freiburg10_Transfection_protocoll.pdf]]<br>
| + | |
| - | Three 10 cm cellcluture dishes with 293 cells were transfected with 10 µg DNA (3,33 µg each plasmid) and the other three 10 cm cellculture dishes with 30 µg DNA (10 µg DNA each plasmid).
| + | |
| - | | + | |
| - | Plasmids:
| + | |
| - | * pAAV_iGEM-MCS_mVenus (P41), 507 ng/µl
| + | |
| - | * pAAV_RC , 1000 ng/µl
| + | |
| - | * pHelper , 280 ng/µl
| + | |
| - | | + | |
| - | Investigators: Patrick, Johannes
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Preparing Viral Stocks</u></p>
| + | |
| - | | + | |
| - | ... according to the standart protocol (see AAV Helper-free System manual). No dilution was performed.
| + | |
| - | | + | |
| - | Investigators: Johannes, Patrick
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>HT1080 Transduction</u></p>
| + | |
| - | | + | |
| - | Four 10 cm cellculture dishes with HT1080 cells have been provided for the transduction.
| + | |
| - | *1 ml of the AAV-2-mVenus (derived from P 39) was pipeted to into dish 1. Note: the virus solution (buffer) showed a yellow coloration.
| + | |
| - | *1 ml of the AAV-2-mVenus (derived from P 39) was pipeted to into dish 2. Note: the virus solution showed (buffer) an orange coloration.
| + | |
| - | *1 ml of the AAV-2-mVenus (derived from P 38) was pipeted to into dish 3. Note: the virus solution showed (buffer) an orange coloration.
| + | |
| - | *1 ml of the AAV-2-mVenus (derived from P 38) was pipeted to into dish 4. Note: the virus solution showed (buffer) an orange coloration.
| + | |
| - | | + | |
| - | Apart from the dilution, the transduction was performed according to the standart protocol.
| + | |
| - | | + | |
| - | Investigators: Johannes, Patrick
| + | |
| - | | + | |
| - | ===38.Labortag 22.06.2010: Midiprep of pHelper plasmid; cloning of the rfc25 MCS===
| + | |
| - | | + | |
| - | Investigators: Bea, Chris W, Adrian, Achim
| + | |
| - | | + | |
| - | *Hybridisation of o'''ligos RFC25 EcoR1+BglII for & RFC25 EcoR1+BglII rev'''
| + | |
| - | **Programm ORIGAMI1 was modified for normal oligos:
| + | |
| - | ***1 95°C 7'
| + | |
| - | ***2 95°C 1'
| + | |
| - | ***-1°C R=0,3°s
| + | |
| - | ***Rep 70
| + | |
| - | | + | |
| - | *conc of Oligos: 138,3µg/µl
| + | |
| - | | + | |
| - | <br> '''Digestion'''
| + | |
| - | | + | |
| - | | + | |
| - | <li> plasmid: name: pAAV_MCS number: P9 production date: 01.06.2010 origin: Adrian, Hanna, Bea
| + | |
| - | <li> new vector name: pAAV_RFC25
| + | |
| - | <li> buffer used: 3 ; Restriction-enzymes used: Enzyme 1 (no. Lab:23) EcoRI ; Enzyme 2 (no.Lab:15) BglII
| + | |
| - | <li> DNA concentration (vector): 261,6 ng/µL | + | |
| - | | + | |
| | | | |
| | {| border="1" | | {| border="1" |
| - | | components || align="right" |volume of vector /µl || align="right" |volume of insert /µl | + | | components || align="right" | V (pCMV_mVenus_YFP)/ µl ||align="right"| V(pAAV_MCS) / µl |
| - | |-
| + | |
| - | | DNA || align="right" | 5,8|| align="right" | none
| + | |
| | |- | | |- |
| - | | BSA (100x) || align="right" | none || align="right" | none | + | | DNA || align="right" | 4.2||align="right"|3.5 |
| | |- | | |- |
| - | | Buffer 3 (10x)|| align="right" |3 || align="right" | none | + | | BSA (10x) || align="right" |3||align="right"|3 |
| | |- | | |- |
| - | |Enzyme EcoRI (no.Lab:23)|| align="right" |1,5 || align="right" | none | + | | Buffer 4 (10x)|| align="right" |3||align="right"|3 |
| | |- | | |- |
| - | |Enzyme BglII (no.Lab:15)|| align="right" |1,5|| align="right" | none | + | |Enzyme: NotI (no.Lab:159)|| align="right" |1,5||align="right"|1,5 |
| | |- | | |- |
| - | |H<sub>2</sup>O|| align="right" | 18,2|| align="right" | none | + | |H2O|| align="right" |18,3||align="right"|19 |
| | |- | | |- |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 30|| align="right" | none | + | |'''Total volume'''|| align="right" |<b>30</b>||align="right"|<b>30</b> |
| | |} | | |} |
| | | | |
| - | <li> Incubation: 1,5 h | + | <li> Incubation: 1 1/2 h at 37°C |
| | | | |
| - |
| |
| - | *A 1% agarose gel was prepared for plasmid separation
| |
| - |
| |
| - | * Production of antibiotika-stock-solutions (10 x 1ml AMP)
| |
| - |
| |
| - | * Midi Prep of pAAV-Helper: concentration. 732,6 µg/µl
| |
| - |
| |
| - |
| |
| - |
| |
| - | Ligation of Oligos: RFC25 and pAAV-MCS.
| |
| - |
| |
| - | * dilution of our Oligos RFC from 138,3 µg/µl 1:10 13,83 (71bp long)=> 0,64µl
| |
| - | * Vektor pAAV-MCS 23.3 µg/µl => 8,36µl
| |
| - | * two plates are placed in the 37°C room
| |
| - |
| |
| - | *Trafo has been performed following standard protocols
| |
| - |
| |
| - | ===39.Labortag 23.06.2010: Preparation of Viral Stock, Seeding cells===
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Design of Primer</u></p>
| |
| - | Achim, Hanna
| |
| - |
| |
| - | In order to sequence the hGH (polyadenylation) sequence and in general the GOI (gene of interest :) ) primer were designed: [[File:Primer GOI+polyA pAAV iGEM-MCS mVenus YFP.pdf]]
| |
| | <br> | | <br> |
| - | These primer will be used in order to sequence P38 (pAAV_iGEM_mVenus-YFP) which delivered fluorescencing HT1080 cells - in comparison to the already sequenced P39 clone. Further on they can be used in general for sequencing any GOI. <br>
| + | 1% Agarose gel |
| - | These oligos were ordered at sigma aldrich as E@sy Oligos.
| + | |
| | <br> | | <br> |
| - | P38 was send for sequencing with GATC_std_pTESp-1 primer: 12 µL P39 (180 ng/µL) + 18 µL H<sub>2</sup>O
| + | <li>1% agarose gel was repared, gel ran for 45 minutes(119 V) |
| | <br> | | <br> |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Sequencing of pKEX-VP1</u></p> | + | <font color=#FF00FF>Results: unexpected sizes of fragments (see protocol), try again next day with an ethidium bromid gel</font> |
| - | Achim, Hanna, Adrian, Chris W.
| + | |
| | <br> | | <br> |
| - | Primer:
| |
| - | * VP1 primer for pKEX forward: V = 3 µL + 27 µL H<sub>2</sup>O
| |
| - | * VP1 primer for pKEX reverse: V = 3 µL + 27 µL H<sub>2</sup>O
| |
| - | Vector:
| |
| - | * pKEX-VP1 (P27.2): c = 693 ng/µL, V = 3 µL + 27 µL H<sub>2</sup>O
| |
| | <br> | | <br> |
| - |
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Thaw HT1080 and AAV-293 cells </u></p>
| |
| - | Achim, Hanna (guided by Sven)<br>
| |
| - | Thawing of HT1080 and AAV-293 cells was performed following this protocol: [[Media:Freiburg10 Thawing cells.pdf]]
| |
| | <br> | | <br> |
| | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Production of chemical competent E.coli</b></p>==== |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Picking clones of pAAV_RFC25 and inoculating 250 mL DYT with pAAV_iGEM_mVenus-YFP (P38) for Midi-Prep </u></p> | + | <p><b>Investigators: Patrick and Jessica</b></p> |
| - | Achim, Chris W., Hanna<br>
| + | <br> |
| - | Bacteria cultures are stored in 37°C room over night.
| + | competent E.coli XL-1-blue and competent E.coli BL21 produced according to the standard-protocoll [[Media:production of competent E.coli.pdf]]<br> |
| | + | <br> |
| | <br> | | <br> |
| | | | |
| - | Adrian, Sven
| |
| - | <gallery widths=500px heights=300px perrow=2 caption="Results of transduction, ~ 1% of the HT1080 showed expression of mVenus.">
| |
| - | File:YFP-Transduction-1.jpg|Bright light image 1
| |
| - | File:YFP-Transduction-2.jpg|Fluoresce microscopy image 1
| |
| - | File:YFP-Transduction-3.jpg|Bright light image 1
| |
| - | File:YFP-Transduction-4.jpg|Fluoresce microscopy image 1
| |
| - | </gallery>
| |
| | | | |
| | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Design of MCS-Oligos</b></p>==== |
| | + | <p><b>Investigators: Bea, Adrian, Hanna, Sven</b></p> |
| | + | <br> |
| | + | In order to replace the multiple cloning site of the pAAV_MCS vector from Stratagene by RFC25, two oligos were designed. After their hybridization the overhangs correspond to ClaI- and BglII restriction sites. A digestion with BglII and ClaI will be performed with pAAV-MCS and then will be ligated with the designed oligos.<br> |
| | + | The oligos (see link) were ordered at Sigma-Aldrich. Estimated shipment: 31.05.2010 .<br> |
| | | | |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Preparation of viral stocks</u></p>
| + | [[File:Freiburg10 Oligos MCS RFC25 for pAAV.pdf]] |
| - | Johannes, Bea
| + | |
| - | Please fill in!!!
| + | |
| - | <br>
| + | |
| - | Steril filtration of virus particles was performed (guided by Sven). Viral stocks are stored at -80°C.
| + | |
| | | | |
| - | ===40.Labortag 24.06.2010: Labmeeting, Presentation/Update by Patrick and Adrian, Midi Prep of pAAV_iGEM_mVenus-YFP, Mini Prep pAAV_RFC25=== | + | ===15. Labortag 26.05.2010=== |
| | | | |
| - | Delivery of:
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Oligos (PstI + MCS)</b></p>==== |
| - | <br> | + | |
| | | | |
| - | Proteinkinase K (will be used for the degradation of the viral capsid prior to qPCR)
| + | <p><b>Investigators: Bea, Hanna, Jessica</b></p> |
| - | <br> | + | |
| - | Benzonase (will be used fur viral stock preparation)
| + | |
| - | <br> | + | |
| - | Primer for for ITR-Conversion to iGEM-Standart
| + | |
| | | | |
| | + | <ul> |
| | + | <li>Repeat cloning of pAAV-MCS + pCMV_mVenus_YFP --> <b>Goal: pAAV_mVenus_YFP</b><br> |
| | | | |
| - | *Presentations of Adrian and Patrick: [[File:Adrian.ppt]] [[File:Patrick.ppt]]
| + | Cloning did not work (see lab day 25.05.2010) either with NotI or with NotI-HF. Gel did not show any proper band at the expected size. <br> |
| | + | There will be used EtBr instead of gelred in the agarose gel and the incubation time of digestion will be prolonged. Further details are followed. <br> |
| | + | NOTE: There are three restriction sites of NotI in the pCMV-mVenus_YFP. If cloning with NotI, the polyA hGH signal will be deleted. therefore cloning with NotI is '''not''' possible . |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Midi-Prep of pAAV_iGEM_mVenus-YFP</u></p> <br>
| |
| - | Investigators: Hanna, Chris W (Adrian inserted RNAse and Ethanol into Buffers)
| |
| - | * new kit from Qiagen was used
| |
| - | * 35 ml of the over-night culture was filled into a 50 mL falcon and centrifuged at '''5000 g''' at '''4°C''' for '''5 minutes'''.
| |
| - | * supernatant was discarded
| |
| - | * Midi-prep was performed according to manual of Qiagen
| |
| - | * final concentrations: 380,4 µg/µl
| |
| - | * Name of eppi: '''P44'''
| |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Mini Prep of P42 and P43 pAAV-RFC-25</u></p> | + | <li>Test digestion of pCMV-mVenusYFP with PstI and MluI <br> |
| - | Investigator: Bea
| + | |
| - | * Mini-Prep was performed following standard protocols
| + | |
| - | * conc: P42: 321,5 µg/µl
| + | |
| - | * conc: P43: 310,1 µg/µl
| + | |
| - | <br> | + | |
| - | ====ITR modification via PCR====
| + | |
| - | *Primers for ITR modification were resuspended and labeled: left forward: o24, left reverse: o25, right forward: o26, right reverse: o27
| + | |
| | | | |
| - | ====Work on pKEX backbones====
| + | <b>Digestion</b> |
| - | Volker
| + | |
| - | | + | |
| - | The sequencing data was evaluated and BLAST searches were carried out on NCBI to answer the question weather the sequence belongs to the backbone pKEX. This question could be answered positively. <br>
| + | |
| - | There are standard Primers from GATC that prime in the sequence. The three expression plasmids from PD Kleinschmidt will therefor be sequenced tomorrow.<br>
| + | |
| - | Additionnaly nine primers for sequences in the AAV Rep and Cap genes were disigned.
| + | |
| - | '
| + | |
| | <br> | | <br> |
| | + | <ul> |
| | + | <li>experiment date: 26.05.2010 </li> |
| | + | <li>plasmid: pCMV_mVenus_YFP; number: P5; production date: 21.05.2010/ pCMV-MSC; number: P2; production date: </li> |
| | + | <li>buffer used: 3/4; Restriction-enzymes used: Enzyme PstI (no. Lab:48) and Enyzme MluI (no. Lab:40) / NotI HF (no. Lab:159) </li> |
| | + | <li>DNA concentration plasmid: P5 477,3 ng/µl / P2 550ng/µl </li> |
| | | | |
| - | ===41.Labortag 25.06.2010: PCR of ITRs, Splitting HT1080 and AAV-293 cells, Seeding of HT1080 cells, Creating Virus Stock Economics, Medium check===
| |
| - | <br>
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Preparation for PCR of ITRs </u></p>
| |
| - |
| |
| - | ==== '''Digestion of pAAV_MCS (P9)''' ====
| |
| - |
| |
| - | <li> experiment date: 25.06.2010
| |
| - | <li> name of investigator: Bea
| |
| - | <li> plasmid: name: pAAV_MCS number: P9 production date: 08.05.2010 origin: SH
| |
| - | <li> buffer used: 4 ; Restriction-enzymes used: Enzyme 1 (no. Lab:113) NgoMIV ; Enzyme 2 (no.Lab:120) AlwNI
| |
| - | <li> DNA concentration (vector): 267,9 ng/µL
| |
| - | <br>
| |
| - | In order to receive the best PCR-results, two digestion reactions/approaches have been performed. <br>
| |
| - | The first digestion was cut only with AlwNI, the second digestion was cut with AlwNI and NgomIV.<br>
| |
| - | <br>
| |
| | {| border="1" | | {| border="1" |
| - | | components || align="right" |v(pAAV_MCS 1) /µl || align="right" |v(pAAV_MCS 2) /µl | + | | components || align="right" | V (pCMV_mVenus_YFP)/ µl ||align="right"| V(pCMV_MCS) / µl |
| | |- | | |- |
| - | | DNA || align="right" | 4|| align="right" | 4 | + | | DNA || align="right" | 4,2||align="right"|3,6 |
| | |- | | |- |
| - | | BSA (100x) || align="right" |none || align="right" | none | + | | BSA (10x) || align="right" |2||align="right"|2 |
| | |- | | |- |
| - | | Buffer 4 (10x)|| align="right" | 2|| align="right" | 2 | + | | Buffer 3 (10x)|| align="right" |2||align="right"|2 |
| | |- | | |- |
| - | |Enzyme AlwNI (no.Lab: 120)|| align="right" |1 || align="right" | 1 | + | |Enzyme: PstI/NotI HF (no.Lab:48/159)|| align="right" |1||align="right"|2 |
| | |- | | |- |
| - | |Enzyme NgoMIV (no.Lab:113)|| align="right" | none || align="right" | 1 | + | |Enzyme: MluI (no.Lab:40)|| align="right" |1||align="right"|- |
| | |- | | |- |
| - | |H<sub>2</sup>O|| align="right" | 13,0|| align="right" | 12,0 | + | |H2O|| align="right" |9,8||align="right"|10,4 |
| | |- | | |- |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | '''20'''|| align="right" | '''20''' | + | |'''Total volume'''|| align="right" |<b>20</b>||align="right"|<b>20</b> |
| | |} | | |} |
| | <br> | | <br> |
| - | <li> Incubation:1,5h | + | <li> Incubation: 1 h at 37°C |
| - | <li> 1% agarose gel has been prepared. Running the gel at 110 V for 45 minutes.
| + | |
| - | <li> Loading plan: Marker (8 µL), pAAV_MCS 1 (24 µL), paav_MCS 2 (24 µL)
| + | |
| - | <br>
| + | |
| | | | |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Splitting of HT1080 and AAV-293 cells, Seeding of HT1080 cells </u></p>
| |
| - | Investigator: Hanna <br>
| |
| - |
| |
| - | '''HT1080:'''
| |
| - | * Cells showed 80 % confluence
| |
| - | * Cells were washed with PBS, detached with Trypsine
| |
| - | * 10 mL DMEM was added, cell suspension was centrifuged for '''5 minutes''' at 200 g.
| |
| - | * Supernatant was discarded, pellet was resuspended with 8 mL DMEM.
| |
| - | * '''Cells were counted: 47.5 µL Trypan blue (stains dead cells) and 2.5 µL cell suspension were mixed and applied onto a Neubauer counting chamber''' -> 2.2 x 20 x 10^-4 cells per mL
| |
| - | * Therefore 27 µL of the cellsuspension was added to each 3 mL DMEM in the 6-well plates. '''-> 2 6-well plates were prepared for transduction!'''
| |
| - | * Further on cells were splitted: 110 µL cell suspension was pipetted into 2 75T flasks each containing 20 mL DMEM and stored in the 37°C incubator.
| |
| | <br> | | <br> |
| - | | + | 1% Agarose gel |
| - | '''AAV-293 cells:'''
| + | |
| - | * Cells showed ~ 50% confluence
| + | |
| - | * Cells were splitted following the standard protocol.
| + | |
| | <br> | | <br> |
| - | | + | <li>1% agarose gel was repared, gel ran for 45 minutes(119 V) |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Creating Virus Stock Economics</u></p> | + | |
| - | Investigators: Adrian
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Medium check </u></p>
| + | |
| - | Investigator: Adrian
| + | |
| - | *three little culture bottles got prepared (H-DMEM, DMEM and PBS) and set into the incubator. In the following days they'll get checked for bacerial growth.<br>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Sequencing results of pAAV_RFC25 </u></p>
| + | |
| - | investigators: Adrian, Bea, Hanna<br>
| + | |
| - | | + | |
| - | [[File:Freiburg10 SeqAnalysis pAAV RFC25 25 06 2010.jpg|900px|thumb|left|SpeI restriction site deletion in sequenced data (on bottom).]]
| + | |
| | <br> | | <br> |
| - | <p style="clear:both;">'''sequenced sample''': pAAV_RFC25 clone 2 (P43)</p>
| + | Results: Test digestion of pCMV_MCS with NotI delivered plausibel results (expected fragment size: ~ 27 kbp and 17 kbp). |
| - | '''used primer''': GATC_std_pTeSp-1.ab1 <br>
| + | Unfortunately the digestion of pCMV_mVenus_YFP with PstI and MluI resulted in one 2.9 kbp and one 1.2 kbp fragment, which didn't correspond to the expected fragment sizes of 19 kbp and 33 kbp - but to fragment sizes of pCMV_MCS without mVenus_YFP! |
| - | The alignment with the theoretical construct showed, that there's a exchange of one base in the RFC25-multiple cloning site. This substitution leads to the deletion of the SpeI restriction site. <br>
| + | |
| - | Therefore we checked the order sheet (everything OK!).<br>
| + | |
| - | We sent the second clone (P42) for sequencing (Hanna).
| + | |
| - | * V(Plasmid) = 6.5 µL
| + | |
| - | * V(H<sub>2</sup>O) = 23.5 µL
| + | |
| - | * Primer: pTESP-1
| + | |
| - | | + | |
| | <br> | | <br> |
| - | <br>
| |
| - |
| |
| - | ====Annotation and studies of the AAV structure====
| |
| - | Investigator: Volker
| |
| - |
| |
| - | The NGR- and the HPSG motif were annotated in the gene sequences according to [[Media: Freiburg10_Michelfelder Trepel 2009 AAV and Their Redirection to Cell-Type Specific Receptors.pdf|[Michelfelder & Trepe; 2009]]]
| |
| - | The pdb sequence were visualized with the programm [http://www.pymol.org| PyMOL] and the important motifs were highlighted.
| |
| - |
| |
| - | <gallery widths=500px heights=300px perrow=2 caption="Impressions from PyMOL">
| |
| - | File:Freiburg10_The viral structure of AAV-2.png|thumb|left| The viral structure of AAV-2
| |
| - | File:Freiburg10_Sturcture of a single AAV-2 VP2 monomer.png|900px|thumb|left| Sturcture of a single AAV-2 VP2 monomer
| |
| - | File:Freiburg10_B-Factor indicating mobility in the crystal.png|900px|thumb|left| B-Factor indicating mobility in the crystal
| |
| - | File:Freiburg10_HPSG-binding-motif.png|900px|thumb|left| HPSG-binding-motif
| |
| - | File:Freiburg10_Residues of the HPSG-binding-motif zoom.png|900px|thumb|left| Residues of the HPSG-binding-motif
| |
| - | File:Freiburg10_NGR-motif.png|900px|thumb|left| The NGR-motif that is responsible for the integrin a5β1 binding
| |
| - | </gallery>
| |
| | <br> | | <br> |
| | <br> | | <br> |
| | + | <li>'''Ordering oligos:'''(Sigma-Aldrich) <br> |
| | + | (Bea)<br> |
| | + | Oligos have been ordered for modifying the ITRs. Deletion of PstI restriction site in ITR. <br> |
| | | | |
| - | ===42.Labortag 26.06.2010===
| |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Transduction of HT1080 cells</u></p>
| + | '''right ITR of pAAV_MCS''' <br> |
| - | Investigator: Adrian
| + | |
| | | | |
| - | * Transduction of 2x6 wells was successfully done (14.25)
| + | oligos ordered:<br> |
| - | * one six well is transduced with 10µg the other with 30µg DNA amount (see HEK293 infection protokoll for further information)<br>
| + | |
| - | (A is up)
| + | fwd: 5´- gcgcagctgcctgcaCGGGCGCCTGATGCGG -3´ 77 °C, 31 bp <br> |
| - | {| border="1"
| + | rev: 5´- CCGCATCAGGCGCCCGTGCAGGCAGCTGCGC -3´ <br> |
| - | | stock solution || align="right" |1:10 Dilution || align="right" |1:50 Dilution
| + | |
| - | |-
| + | |
| - | | 1:100 Dilution || align="right" | 1:500 Dilution|| align="right" | control (500µl DMEM)
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br> | + | |
| - | *the delution steps (1:10, 1:50, 1:100, 1:500) are placed upon the virus stocks in the -80°C freezer in 15 ml falcons.
| + | |
| | <br> | | <br> |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Did primers for sequencing GOI arrive?</u></p> | + | '''leftITR of pAAV_MCS'''<br> |
| | | | |
| - | ====Paper reading session====
| + | oligos ordered:<br> |
| - | Investigators: Igor, Anna, Patrick, Stefan and Volker
| + | |
| - | | + | fwd: 5´- CCTTTTGCTCACATGTCGTGCAGGCAGCTGCGCG -3´ 74 °C, 34 bp <br> |
| - | Several papers were read and infomation on cap-modifications were extracted.
| + | rev: 5´- CGCGCAGCTGCCTGCACGACATGTGAGCAAAAGG -3´<br> |
| | + | <br> |
| | + | For further details see link <br> |
| | | | |
| - | *Trasitions of seven Tyrosines (252, 272, 444, 500, 700, 704 and 730) to Phenylalanines as described in [[Media:Freibur10_Next_generation_of_adeno-associated_virus_2_vectors-_point_mutations_in_tyrosines_lead_to_high-efficiency_transduction_at_lower_doses.pdf|[Zhong et al. 2008]]]
| + | http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/images/4/44/Freiburg10_Oligos_ITR_mutagenesis_for_pAAV_delete_PstI.pdf |
| - | *Transitions of two amino acids (R459D and N551D) leading to reduced sero prevalence as described in [[Media:Designer_Gene_Delivery_Vectors-_Molecular_Engineering_and_Evolution_of_Adeno-Associated_Viral_Vectors_for_Enhanced_Gene_Transfer.pdf|[Inchan Kwonand, David V. Schaffer]]]
| + | </li> |
| - | [[File:Freiburg_Results from the journal club at the 26th june.png|900px|thumb|left|Proposed transitions Y252F, Y272F, Y444F, Y500F, Y700F, Y704F and Y730F in red & R459D and N551D in green]]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br> | + | |
| | <br> | | <br> |
| | | | |
| - | ===43.Labortag 28.06.2010: Checking HT1080 for YFP-Expression, Creating plan: urgent things to do===
| + | '''Test-Trafo of chemical competent E.coli cells''' <br> |
| | + | (Hanna) |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Checking HT1080 for YFP-Expression </u></p>
| + | The competent E.coli XL-1-blue and competent E.coli BL21 which were prepared the day before were test-transformed with pUC18 - following the standard protocol. Cells were plated on agar plates containing ampicillin and stored over night at 37°C. |
| - | *Hannas 2*6 wells (24.6) transduced by (Adrian 25.6)
| + | Further on two control plates containing ampicillin or kanamycin were prepared. Non-transformed cells were plated on them and also stored over night at 37°C. |
| - | Investigator: Sven, Patrick, Adrian
| + | |
| - | | + | |
| - | <gallery widths=500px heights=300px perrow=2 caption="Results of transduction nr.2">
| + | |
| - | File: Freiburg10_2Transd10µg_unverd_2_(c1).JPG|10µg_unverd_2_(c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd10µg_unverd_1_(c1).JPG|10µg_unverd_1_(c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd30µg_unverd_2_(c1).JPG|30µg_unverd_2_(c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd30µg_unverd_(c1).JPG |30µg_unverd_(c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd30µg_unverd_4_(c1).JPG|30µg_unverd_4_(c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd30µg_unverd_3 (c1).JPG|30µg unverd 3 (c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd30µg_unverd_6_(c1).JPG|30µg_unverd_6_(c1).JPG 48h post transduction
| + | |
| - | File: Freiburg10_2Transd30µg_unverd_5_(c1).JPG|30µg_unverd_5_(c1).JPG 48h post transduction
| + | |
| - | | + | |
| - | | + | |
| - | </gallery>
| + | |
| - | * The transduced cells will be checked again (29.6.).
| + | |
| | <br> | | <br> |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Sequencing results of P42</u></p>
| |
| - | Hanna <br>
| |
| - | Sequencing of pAAV_RFC25 (P42):
| |
| - | [[File:Freiburg10 P42.jpg]]
| |
| - | Sequencing delivered, that there weren't any base exchanges or deletions in this clone. Therefore P43 was dismissed (Plasmid and glycerol stock). <br>
| |
| | <br> | | <br> |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Theoretical construction of our biobricks</u></p>
| + | </ul> |
| | + | </ul> |
| | + | ===16. Labortag 27.05.2010=== |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Theoretical cloning</u></p> | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Cell counting</b></p>==== |
| - | Investigators: Hanna, Adrian, Chris W, Patrick, Volker
| + | |
| | | | |
| - | Urgent Things to do:
| + | <p><b>Investigator: Patrick, Adrian, Chris W. Christian L.</b></p> |
| | | | |
| - | *Modification CAP: | + | * Meeting |
| - | **HSPG -> 2 AS
| + | |
| - | **Targeting (585,588...) -> 2 AS -> 1 or 2 restriction sites?! Investigator: Volker
| + | |
| - | ** Seroprevalence -> 2 AS
| + | |
| - | ** thyrosine for ubiquitination Y252F, Y272F, Y444F, Y500F, Y700F, Y704F and Y730F
| + | |
| - | **Primer: start codons
| + | |
| - | **EGF-Rezeptor: A431-Zellinie
| + | |
| - | ***Affibody: order the Gensequence
| + | |
| - | ***bispecific AK -> order Diabodys (are there any restriction sites?) Investigator: kristian???
| + | |
| - | <br>
| + | |
| - | *Ordering the ITRs Investigator: Hanna
| + | |
| - | **Stratagene: right and left
| + | |
| - | **wild-type
| + | |
| - | <br>
| + | |
| - | *Modification: REP Investigator: Volker
| + | |
| - | ** 3 restriction sites -> site directed mutagenisis vs. synthetic? ->order (check which is the cheaper approach)
| + | |
| - | <br>
| + | |
| - | *HGH Investigator: Hanna
| + | |
| - | ** restriction sites? -> are there any available biobricks?
| + | |
| - | **biobrick production
| + | |
| - | <br>
| + | |
| - | *Beta-globin: what is the function? -> cut out?
| + | |
| - | **where is the start and the end?
| + | |
| - | **restriction sites?
| + | |
| - | **biobrick production
| + | |
| - | <br>
| + | |
| - | *GMK-TK
| + | |
| - | **restriction sites: primer
| + | |
| - | **biobrick production
| + | |
| - | **ordering the SR39
| + | |
| - | <br>
| + | |
| - | *tumor specific promotors
| + | |
| - | **more Information!!!
| + | |
| - | ** telomerase specific promoters!!!
| + | |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Continuation of ITR PCR</u></p>
| + | |
| - | Hanna, Chris W., Adrian <br>
| + | |
| - | * Analytic gel was run: 1.7 µL loading dye (6x) was added to 8.3 µL of each sample (left ITR: 5 µL DMSO + 10 µL DMSO; right ITR: 5 µL DMSO + 10 µL DMSO).
| + | |
| - | * 110 V, 35 minutes
| + | |
| - | * No ITr bands were detectable - just 2 weak "primer-bands"
| + | |
| - | Conclusion: PCR didn't work. Because of that new primers were designed and ordered (Achim), which are longer and posses therefore higher annealing temperatures.
| + | |
| - | <br>
| + | |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>ITRs (theoretical)</u></p>
| + | cell counting: |
| - | Adrian, Achim, Hanna <br>
| + | |
| - | * trs sequences (GTTGG) are present in both ITRs (Stratagene)!
| + | |
| - | * assumption: transcription despite of ITR-secondary structure - no single-strand nick at trs-sequence in transduced cells ?
| + | |
| | <br> | | <br> |
| - | | + | * BL21 + PUC18 Trafo 54*4 = 216 |
| - | ===44.Labortag 29.06.2010: Discussionround 3, Seeding HEK293 cells for transfection and titering ===
| + | * XL1B + PUC18 Trafo 30*4 = 120 |
| - | | + | digitalization of recipe-cards (Christian L.) |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>DISCUSSIONROUND</u></p>
| + | |
| - | | + | |
| - | *Affibodys | + | |
| - | **Investigator: Anna | + | |
| - | ***Affibodys are 6-7kD Proteins derived from Protein A/Z
| + | |
| - | ***they are binding to domain 3 from IGFR
| + | |
| - | ***13 AS are replaceable for changing the tropism
| + | |
| | <br> | | <br> |
| - | *Tyrosine Mutants
| |
| - | **Investigator: Adrian
| |
| - | ***replacements are done in VP3! => Volker?!
| |
| - | ***no multi-mutants are available (instead of single mutations many Y->F)
| |
| - | ***best candidates are: Y730F and Y444F.
| |
| - | <br>
| |
| - | *Second strand DNA-synthesis in Transduced cells
| |
| - | **Investigator: Achim
| |
| - | *** Dual strand synthesis is the main limiting for transduction/transgen expression.
| |
| | | | |
| - | <br>
| + | ===17. Labortag 31.05.2010=== |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Seeding HT-cells for transfection and titering</u></p>
| + | |
| - | Investigators: Sven, Adrian, Bea
| + | |
| - | *The pellet was resolved in 4ml we had a 550.000 cells/ml
| + | |
| - | * 2x6 well dishes got prepared:
| + | |
| | | | |
| - | 1. Plate I(A is up)
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>pAAV_RC R585A and R588A transistions</b></p>==== |
| - | {| border="1"
| + | |
| - | | 3x10^5 cells || align="right" |3x10^5 cells || align="right" |3x10^5 cells
| + | |
| - | |-
| + | |
| - | | 3x10^5 cells || align="right" | 3x10^5 cells|| align="right" | 3x10^5 cells
| + | |
| - | |-
| + | |
| - | |}
| + | |
| | | | |
| - | <br>
| |
| - |
| |
| - | 2. Plate II (A is up)
| |
| - | {| border="1"
| |
| - | | about 3x10^5 cells || align="right" |only medium|| align="right" |only medium
| |
| - | |-
| |
| - | | about 3x10^5 cells || align="right" |only medium|| align="right" |only medium
| |
| - | |-
| |
| - | |}
| |
| - | <br>
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Seeding HT1080-cells for transduction and qPCR</u></p>
| |
| - | Hanna <br>
| |
| - | * Cell density was determined with trypan blue and counting chamber: 2 x 20 x 10^4 cells/mL
| |
| - | * wanted: 5 x 10^4 cells --> 125 µL cell suspension per well
| |
| - | * Cells were seeded into a 24-well-plate (row C and D!): 1.5 mL DMEM + 125 µL cell suspension per well
| |
| - | * cells were incubated for 1 hour at 37°C
| |
| - | <br>
| |
| - | Further on, cells were splitted: ~ 3 mL cell suspension was added to ~ 17 mL DMEM
| |
| - | <br>
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Transduction of HT1080 cells for qPCR</u></p>
| |
| - | Chris W. <br>
| |
| - |
| |
| - | ===45.Labortag 30.06.2010: Titering Procedure via qPCR, Transduction of 6well plates nr.4, (Seeding HEK293 for Transfection?), further Gene-Order-Investigation ===
| |
| - |
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Harvesting cells for qPCR</u></p>
| |
| - | Investigators: Chris W, Hanna <br>
| |
| - | Transduced HT1080 cells were harvested following Sven's standard protocol.
| |
| - |
| |
| - | <br>
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: blue;"><u>Transduction of 2 x 6well plates nr.4</u></p>
| |
| - | Investigators: Bea, Adrian...
| |
| - |
| |
| - | *Master plan: Different Transduction Mechanisms
| |
| - | **rise the amount of transduced particels, instead of 500µl, 1000µl of the AAV-stock getting pipetted into each well.
| |
| - | **the second plate gets stimulated via UV-light to boost the expression of DNA-Repair mechanisms. This should enhance the second strand synthesis of our ssDNA-GOI => a rise of YFP Expression!
| |
| - |
| |
| - | 1. Plate I(A is up)
| |
| - | {| border="1"
| |
| - | | 3x10^5 cells || align="right" |3x10^5 cells || align="right" |3x10^5 cells
| |
| - | |-
| |
| - | | 3x10^5 cells || align="right" | 3x10^5 cells|| align="right" | 3x10^5 cells
| |
| - | |-
| |
| - | |}
| |
| - | Transduction:
| |
| - | <br>
| |
| - |
| |
| - | {| border="1"
| |
| - | | 1000 µl of Viral Stock DROPPED || align="right" |1000 µl of Viral Stock gently resuspended|| align="right" |1500 µl of Viral Stock hard resuspending
| |
| - | |-
| |
| - | | 1000 µl of Viral Stock DROPPED || align="right" | 1000 µl of Viral Stock gently resuspended|| align="right" | no Transduction
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | <br>
| |
| - |
| |
| - | 2. Plate II (A is up)
| |
| - | {| border="1"
| |
| - | | about 3x10^5 cells || align="right" |only medium|| align="right" |only medium
| |
| - | |-
| |
| - | | about 3x10^5 cells || align="right" |only medium|| align="right" |only medium
| |
| - | |-
| |
| - | |}
| |
| - | <br>
| |
| - | Transduction:
| |
| - | <br>
| |
| - | {| border="1"
| |
| - | | 1500 µl of Viral Stock gently resuspended || align="right" |no Transduction|| align="right" |no Transduction
| |
| - | |-
| |
| - | | 1500 µl of Viral Stock gently resuspended || align="right" |no Transduction|| align="right" |no Transduction
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>(Seeding HEK293 for Transfection?)</u></p>
| |
| - | Investigators: ...
| |
| - |
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: blue;"><u>beta-globin intron</u></p>
| |
| - | Investigators: Adrian, Bea
| |
| - | <br>
| |
| - | We found some interesting literature which point out that introns have an influence on the eucaryotic gene expression on DNA and RNA level. Next steps are going to be the designing of the beta-globin intron and maybe further analysis of the other mentioned introns. Maybe two or three more introns can be ordered.
| |
| - | <br>
| |
| - | [[Media:Freiburg10_LeHir_et_al_How_introns_influence_and_enhance_eukaryotic_gene_expression_2003.pdf]]
| |
| - | <br>
| |
| - | [[Media:Freiburg10_Noe_et_al_An_intron_is_required_dro_dihydrofolate_reductase_protein_stability_2008.pdf]]
| |
| - | <br>
| |
| - | [[Media:Freiburg10_Mashadrizeh_et_al_A_systematic_study_of_the_function_of_the_human_beta-globin_introns_on_the_expression_of_the_human_coagulation_factor_in_cultured_Chinese_hamster_ovary_cells_2009.pdf]]
| |
| - | <br>
| |
| - | * [[Media:Chicken_beta-globin_insulator_overcomes_variegation_of_transgenes_in_Xenopus_embryos.pdf]]
| |
| - | <br>
| |
| - | <br>
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>hGH sequence</u></p>
| |
| - | Hanna
| |
| - | * orientation in pAAV_MCS is reverse annotated... but in the same orientation as in human growth hormone I gene!
| |
| - | * Blast and alignment with other expression vectors and the human growth hormone I revealed that Stratagene annotated 1 bp too much
| |
| - | * "A common feature of mRNA in higher eukaryotes (but not in yeast) is the presence of the highly conserved sequence AAUAAA in the region from 11-30 nucleotides upstream of the site of poly(A) addition. (...) The signal is needed for both cleavage and polyadenylation." (Genes VIII, Lewin, P. 720)
| |
| - | * good news: There're no iGEM restriction sites in the sequence (RFC25)
| |
| - | * Conclusion: Instead of ordering the whole sequenc in iGEM standard it would be probably easier (and cheaper) to perform a PCR.
| |
| - | * '''Done''': Oligos were designed for PCR!
| |
| - | <br>
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>ITRs: new strategy</u></p>
| |
| - | Hanna <br>
| |
| - | Plan:
| |
| - | * Digest pAAV_MCS with AlwNI. Result: one large fragment containing the left ITR + a small fragment containing right ITR.
| |
| - | * Digest both fragments with NotI and PstI.
| |
| - | * Hybridize oligos containing RFC10 restriction sites (designed by Hanna), ligate them via intermediate steps (ligating into a vector) with ITRs . Comment: One oligo posseses a blunt end - therefore "intermediate step-vector" needs to be digested with an enzyme producing a blunt site.
| |
| - | *''' Done:''' Oligos were designed. Need to be checked!
| |
| - | <br>
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color: fuchsia;"><u>Sequencing of pAAV_iGEM-mVenus-YFP</u></p>
| |
| - | Hanna, Chris W. <br>
| |
| - | * 3 µL of O28, 29 and 30 each were mixed with 27 µL water -> primer fpr sequencing of GOI and of hGH sequence
| |
| - | * 11.1 µL plasmid + 18.9 µL H<sub>2</sup>O
| |
| - | <br>
| |
| | | | |
| - | <p style="font-size:15px; font-weight: bold; color: #014421;"><u>Investigation of the Kleinschmidt sequencing</u></p>
| |
| - | Investigator: Volker
| |
| - | [[File:Freiburg_VP3ex sequencing.png|800px|thumb|right|The CMV promoter sequence was found in front of the expression constructs of VP2 and VP3]]
| |
| | | | |
| - | The sequencing of den unknown backbone pKEX revealed that the construct VP1ex from the DKFZ seemed not to contain a regulatory region for the expresion of the VP1 ORF, but in the backbonesequence there were some standard primers from GATC that could be used to sequence the insert of all expression constructs.<br><br>
| + | In order to alter the tropism of AAV2 several modifications have to be performed. |
| - | This sequencing was done at the 26.06.2010. The read into the construct VP1 showed a long homologous region with the ORF of VP1 where as the sequencing of VP2ex and VP3ex did not show a homologous region with the ORFs of VP2 and VP3. <br><br>
| + | First of all the binding to Heparan Sulfate Proteoglycan has to be disrupted. This can be done by R585A and R588A transistions: |
| - | The test weather primers of GATC bind to the unknown region gave a positive hit in both constructs with the primer GATC_std_CMV_f. This primer is specific for the sequence of the CMV promoter. After this indice the sequences VM_2 26.06. -pBR1.ab1 and VM_3 26.06. -pBR1.ab1 were aligned with the sequence of the CMV promoter provieded in the stratagene kit in the plasmid pAAV-MCS. The majority of the sequence fitted well with CMV promoter as indicated in the image in purple. At the 5' end of the CMV promoter there were some missmatches in the alignment indicated in yellow.<br><br>
| + | [[File:Freiburg10 R585A R588A.jpg|800px|]] |
| - | The fact that VP2ex and VP3ex contained a CMV promoter element which was not included in VP1ex was unexpected. The sequencing with the standard primer GATC_std_CMV_f which binds at the 3' end of the CMV promoter and will probably sequence the ORF of VP2ex and VP3ex was ordered.<br><br>
| + | |
| | | | |
| | <html> | | <html> |
| | </div> | | </div> |
| | </html> | | </html> |

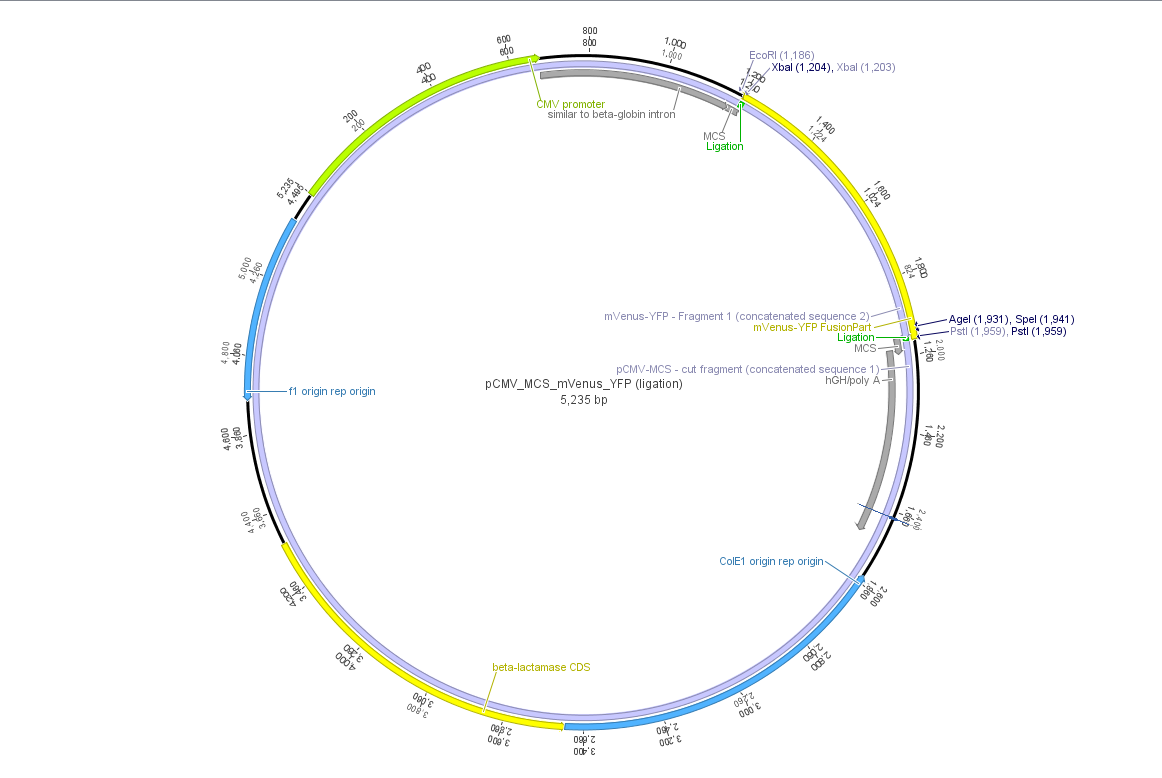

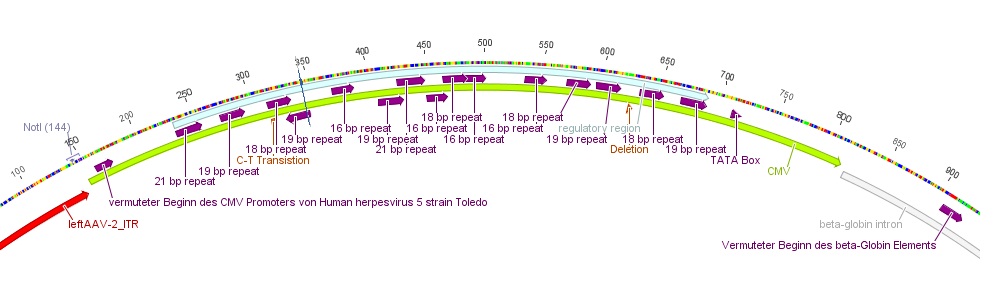
 "
"