Team:Freiburg Bioware/Project/Project Description
From 2010.igem.org
| Line 56: | Line 56: | ||
<div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;border: 1px solid black;"><p> | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;border: 1px solid black;"><p> | ||
<!---Insert text in here---> AAV bears its natural tropism for HSPG on one of its major exposed surface loops - at amino acid position 585/588. Insertions of small binding motives into this region as well as into another surface loop at amino acid position 453 have proved effective for retargeting the virus towards different receptors. <br> | <!---Insert text in here---> AAV bears its natural tropism for HSPG on one of its major exposed surface loops - at amino acid position 585/588. Insertions of small binding motives into this region as well as into another surface loop at amino acid position 453 have proved effective for retargeting the virus towards different receptors. <br> | ||
| - | We introduced two pairs of single cutting restriction sites into each surface loop, allowing an easy swapping of different loop insertions. Our kit comes with a number of those loop insertion motives, labeled ViralBricks. | + | We introduced two pairs of single cutting restriction sites into each surface loop, allowing an easy swapping of different loop insertions. Our kit comes with a number of those loop insertion motives, labeled ViralBricks. We do not only provide ViralBricks for differential targeting and knocking out of the natural tropism, we also included inserts for purification and detection of virus particles. |
</p></div> | </p></div> | ||
<div style="float:right; width:420px; height:auto; "><img src="https://static.igem.org/mediawiki/2010/1/1b/Freiburg10_ViralBrick.png" width="420" | <div style="float:right; width:420px; height:auto; "><img src="https://static.igem.org/mediawiki/2010/1/1b/Freiburg10_ViralBrick.png" width="420" | ||
Revision as of 13:08, 25 October 2010
The Experimental System
Therapy using viral vectors is an promising approach for....
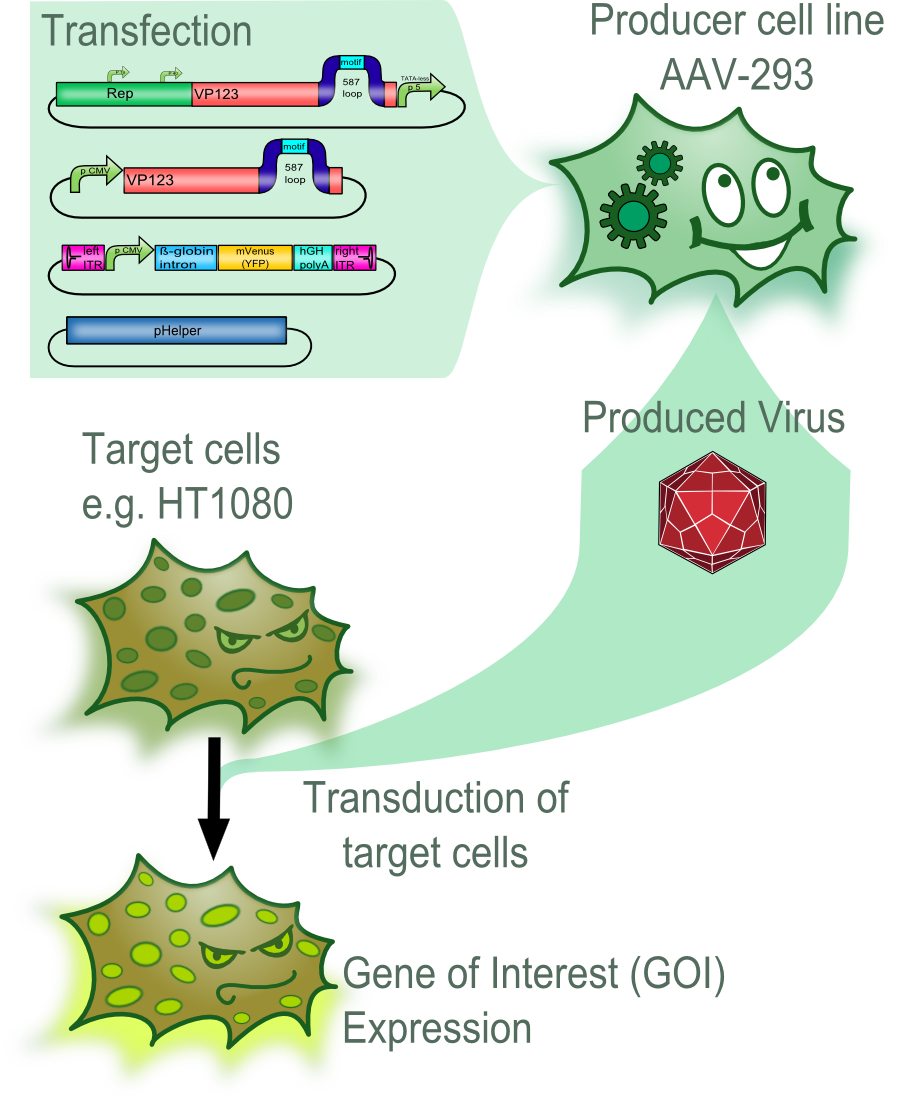
In an first step the plasmids of the AAV-2 Helper-free System were genetically modifyed by converting it into BioBricks and inserting of targeting molecules into the constructs. These plasmids were then used to transfect the producer cell line AAV-293. After an incubation of three days the viral vectors were harvested and used to transduce different target cells. The succesful transduction can then for example be measured by detecting the fluorescence of fluorescent proteins in the target cells.
The majority of the modifications that were introduced into the viral vector aimed to allow differential targeting of tumor cell over healthy off-target cells.

Layers of specificity
Employment of viral vectors for means of therapy is idea in the context of personalized medicie that gets more and more interest. In such applications the reduction of side effects and the safety of the patient in general is of the highest priority.
In order to satisfy this requirement we designed our Therapy Vector with several layers of Specificity:
- The targeting of the viral vector towards the desired target cell (e.g. tumor cells) is the basic idea behind the emplyment of viral vectors for therapeutical means. There for the natural tropismn has to be knocked down and a desired tropism has to be introduced that allows differential targeting of pathological but not of off-target cells. To fulfill this mission our Virus Construction Kit offers you different solutions.
- Off-target cells that were transduced by mistake can be preserved from an undesired therapy effect when the therapeutic gene is controley by a tissue specific promoter. For this mean a promoter has to be used that is as specific for the pathological tissue as possible. We included the human telomerase promoter (phTERT) which is often activated in tumor cells and is there for able to allow differential experssion of a therapeutic geneproduct in pathological cells.
- For reasons of safety Therapeutic vector do not directly trigger appoptosis in the successfully targeted cells. To include one further layer of specificity and safety we decided to arm our therapy vector with different prodrug convertases. Neither the single application of the harmless prodrug nor the single expression of the convertase has a noteworthy effect of the transduced cell. Only in cells that express the prodrug convertase and have a sufficient cytoplasmatic concentration of the belonging prodrug apoptosis is triggered. This dependency of the therapy on a prodrug can be employed to protect tissues or other persons that could come in contact with the therapeutical vector. This aspect was specially inportant for the development of a viral vector that is able to infect humans in the context of a undergraduate project for the iGEM competition. Therefor this approach gained our preference over other possibly equivalent arming possibilities described in the tumor therapy with viral vectors.

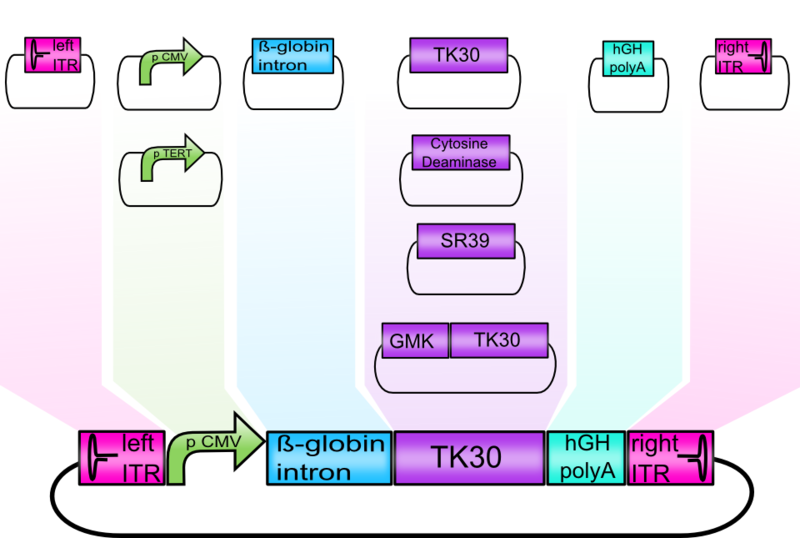
Modularization of the rAAV genome
Text
Modulatization of the RepCap plasmid
Text
Modulatization of the Vectorplasmid
Text

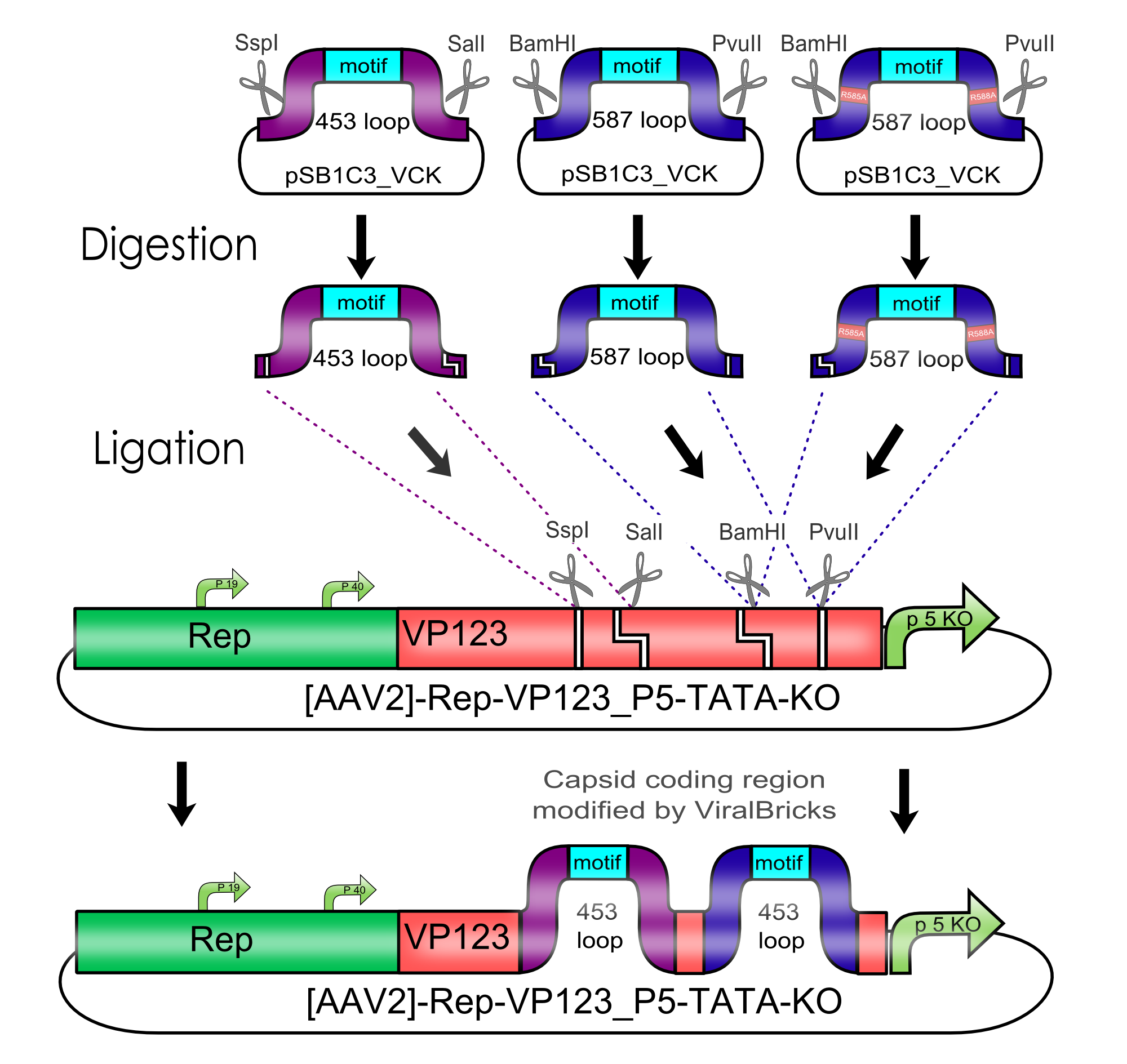
Modification of the viral surface in one cloning step - The ViralBrick standard
AAV bears its natural tropism for HSPG on one of its major exposed surface loops - at amino acid position 585/588. Insertions of small binding motives into this region as well as into another surface loop at amino acid position 453 have proved effective for retargeting the virus towards different receptors.
We introduced two pairs of single cutting restriction sites into each surface loop, allowing an easy swapping of different loop insertions. Our kit comes with a number of those loop insertion motives, labeled ViralBricks. We do not only provide ViralBricks for differential targeting and knocking out of the natural tropism, we also included inserts for purification and detection of virus particles.

Midifications of the Virus Shell via Loop Insertions
Text
Specific biotinylation of the Viral Schell - The Biotinylation Acceptor Peptide (BAP)
Text
Purification of Therapeutic Viral Vectors - The His-Affinity Tag
Text
Targeting of integrin overexpressing cells - The RGD Motif
Text
Arming the Viral Vector with therapeutic antibodies - The Z34C Motif
Text
Testing the limit for loop insertion - The Beta-Lactamase
Text
Method Development
Text
Arming: Killing the Tumor
Text
All-In-One: Testing multiple modified Viral Vectors
Text
Virus Construction Kit - The Manual
Text







 "
"