Team:Freiburg Bioware/Project/Methods
From 2010.igem.org
Methods
Contents |
Method Development
Purification of AAV particles
Experiences from under the hood: Cell Culture
The optimization of the lab intern standard protocols was one of our fields on investigation. Besides optimizing the handling of our different cell lines and working steps, the transfection protocol was examined. One of the most remarkable lessons after several transfection performed was the crucial handling of the AAV293 cells. Once over approximately 80 % confluence the cells are no longer competent for transfection. Another achievement in method development was the determination of the optimal plasmid amounts. The best results were performed using 3.3 µg per plasmid and therefore this parameter was modified in our standard protocol. After transfecting the AAV293 we were able to detect the Ca2+-DNA conglomerates in the medium. The toxic side effects of these conglomerates were also confirmed. Not only the medium had to be changed, but also washing with PBS was essential to keep the cells alive.
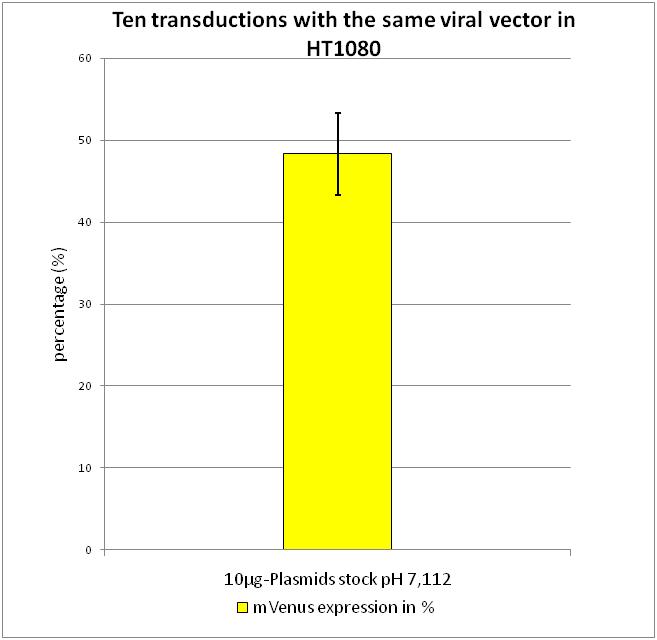
The most critical steps in transfection is the exact pH of the 2x Hepes buffered-saline (HBS) buffer, in which the C2+-DNA complexes precipitate. Our initial buffer had a pH of 7.112. To determine the best pH-value for transfection, transfections were performed using buffers with different pH, harvested the viral particles and used the flow cytometry to define the optimal pH. Transfection, harvesting and transducton were performed according to the modified standard protocol.
After confirming that the highest amount of viral particles was created with the pH 7.112 2xHBS we wanted to determine how valid the flow cytometry data is and created a standard derivation.
After transduction with equal volumes of the created viral stock the flow cytometry was performed according to FACS protocol. As it can be seen there is only a small standard derivation of 6.19% in mVenus positive cells. By combining the results gained from the experiments described above, we were able to evaluate the standard protocol described in Material and Methods.
Imaging AAV: EM/AFM pictures
To visualise our custom AAV particles, we took electron microscopy as well as atomic force microscopy pictures from our virus samples
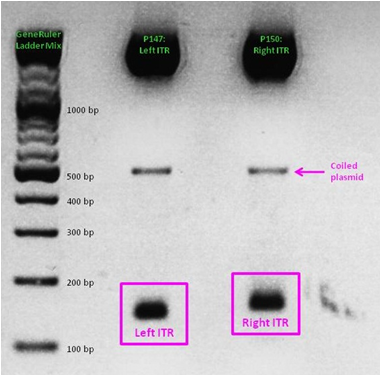
ITR cloning
As a part of our modularization of the AAV vector plasmids, we needed to extract the sequences making up the ITRs at each end of the vector and clone them into an IGEM-compatible backbone. But due to the ITRs’ strong secondary structures, none of our PCR-based approaches worked. External companies weren’t able to synthesize or even sequence the ITRs. Taking advantage of NotI and PstI restriction sites flanking the ITRs, we worked out a complex cloning strategy that finally led to functional ITR motives in the rfc10 standard.
See also: Hannas' ITR Diary
Serum-free cell culture medium
Introduction
Serum-free mediums allow users to standardize their cell culture conditions. It contains no animal proteins or animal-origin constituents, e.g. FCS (fetal calf serum).
The AAV-293 cells are used for AAV-2 production and are usually grown in (among other chemicals, such as nutrients, antibiotics, growth factors) serum supplemented DMEM medium. Regarding Western Blots, size exclusion chromatographies and other (purification) methods, the undefined and also highly variable serum products can disturb or interfere with these methods. Therefore it is useful for many applications to grow AAV-293 cells in serum-free medium.
Because our long term goal for AAV vectors is application in human patients, we are also trying to develop new methods to produce pure, uncontaminated AAV particles. The use of FCS to supplement cell culture medium for AAV particle production is problematic because even smallest amounts of animal antigens in the administered drug could lead to a strong immune response in patients.
Testing serum free medium
Serum free medium was obtained from AAV-293 cells are not adapted to serum-free growth conditions so we had to accustom them to the new growth conditions step by step, starting with 25% serum-free medium, e.g. 15 ml DMEM (not serum-free) + 5 ml serum-free medium (FreeStyle™ 293 Expression Medium) for a T75 flask. Each step takes at least 1 passage. We raised the serum-free ratio to 100 % over 7 passages.
100% serum-free cells grow slower compared to the serum-supplemented ones and we had to check them regularly via microscopy because the medium contains no pH indicator.
Results
Even though cells grew slower and handling was more difficult due to a missing pH indicator, we successfully cultivated AAV-293 cells in serum-free medium. The cells were used for AAV production, and we produced similar amounts of virus particles compared to cells grown in FCS-supplemented medium. Production efficiency can’t be compared exactly because after seeding cells for transfection they don’t grow as fast as the AAV-293 in serum containing medium.
Established Methods
Protocols; Standard Operating Procedures
Standard Protocol: Cloning
Media:Freiburg10_Advanced_Cloning_Protocol_04_08_2010.pdf
Media:Split_cellculture.pdf
Media:Freeze_cellculture.pdf
Media:production of competent E.coli.pdf
Media:Freiburg10_Transfection_protocoll.pdf
Media:Freiburg10 Thawing cells.pdf
Media:Freiburg10_Aminoacids_vs_restrictionsites.pdf
Media:Freiburg10 Endotoxinfreie Midi.pdf
Media:Freiburg10_LB+Agar.pdf
Media:Freiburg10_Subcloning_cap_into_pAAV_RC.pdf
Media:Freiburg10_Quantitative_realtime_PCR_for_Titering_of_infectious_AAV_particles.pdf
 "
"