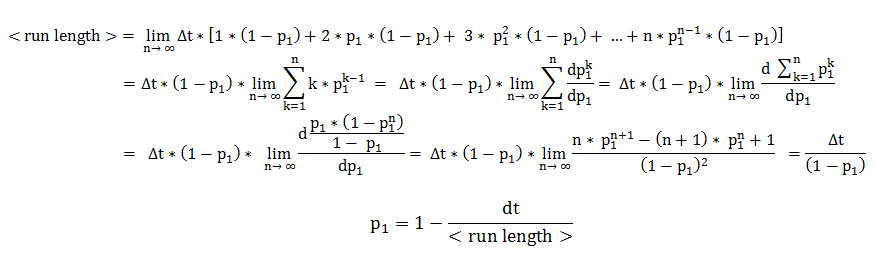Team:ETHZ Basel/Modeling/Movement
From 2010.igem.org
(→Bias) |
(→Algorithm) |
||
| Line 69: | Line 69: | ||
We use a two state model that reflects the two states the cell can be in through out its motion - swimming state & the tumbling state. At each time-step of the simulation, the cell will either stay in the same state or will switch the state according to these probabilities. Details of these probability values can be found below. | We use a two state model that reflects the two states the cell can be in through out its motion - swimming state & the tumbling state. At each time-step of the simulation, the cell will either stay in the same state or will switch the state according to these probabilities. Details of these probability values can be found below. | ||
| - | + | In each of these states, there is a transition probability to switch to the other states as described above. (We have the probability P1 that tells us the probability of switching to the tumbling state when the cell is currently in the swimming state and also the probability P2 that that reflects the probability of switching to the running state). At each timestep of the algorithm, the decision whether to run or tumble in the next timestep is taken based on these two probabilities. | |
| - | In each of these states, there is a transition probability to switch to the other states | + | |
If the decision was to run in the next timestep, the cell will be moved to it's next point with a velocity that agrees with literature [1]. Also, there is a slight deviation in the angle it moves during running. This angle is much smaller than the angle change during a tumbling timestep & it is sampled from a distribution that is suggested based on empirical data [1]. | If the decision was to run in the next timestep, the cell will be moved to it's next point with a velocity that agrees with literature [1]. Also, there is a slight deviation in the angle it moves during running. This angle is much smaller than the angle change during a tumbling timestep & it is sampled from a distribution that is suggested based on empirical data [1]. | ||
| - | During a tumbling timestep, the angle of tumbling is decided such that the tumbling | + | During a tumbling timestep, the angle of tumbling is decided such that the tumbling angle (between two directed motion segments) is a Weibull distributed random variable as suggested in [1]. |
| - | We have made sure that the movement simulated using the algorithm agrees with the observed behaviour of E. coli in | + | We have made sure that the movement simulated using the algorithm agrees with the observed behaviour of E. coli in chemotaxis motion. |
<br> | <br> | ||
Revision as of 21:13, 23 October 2010
Modeling & Simulating Bacterial Movement
While the biologists are busy teaching E. coli how to respond to light, the modelers thought of simulating the soon-to-be E. lemming electronically. This will close the modeling loop of our system and will allow us to check the imaging pipeline, microscope analysis & joystick control even without having the real E. lemming under the microscope.
Overview
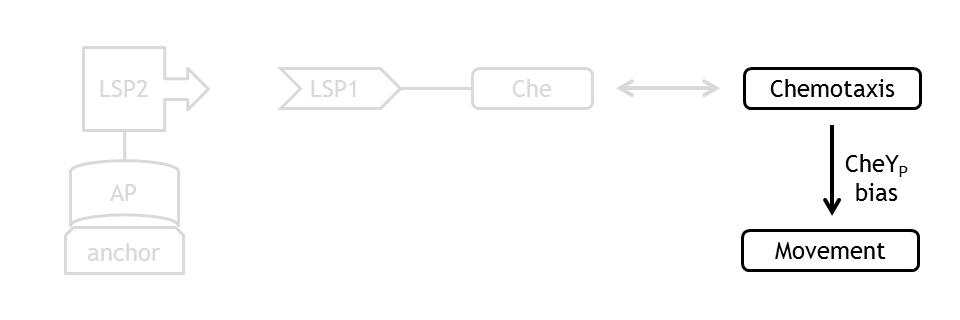
Analogous to the highly complex signal transduction network in the chemotaxis receptor model, there is an equally challenging molecular mechanism on side of the flagella, responsible for the movement of the bacterium.
In order to capture the realistic features of cell motility, we implemented a probabilistic model, which performs in accordance to the existing empirical observations on the chemotaxis movement. At every time point of the simulation, CheYp concentration is received as an input from the chemotaxis pathway model. This input determines the direction (CCW or CW) of rotation of the flagellar motor. A counterclockwise rotation corresponds to the directed movement (running) state and to a change in spatial coordinates, together with a slight change in angle, while a clockwise rotation corresponds to the tumbling state and to a new movement direction, with unchanged spatial coordinates.
The coupling of the probabilistic movement model with the molecular model ensures that we can realistically simulate the variations in motility with the variations in protein concentrations.
Concepts
The main concepts we used in deriving and implementing our model are based on the most important features observed in the real behavior of E. coli. These features are:
Bias
The quantity that links the CheYp concentration with the type of motion (tumbling vs. directed motion) is called the bias and it is formally defined as the fraction of time spent in the directed movement state with respect to the total time in motion. Our choice for bias formulation was a nonlinear Hill - type function of CheYp concentration, with one of the parameters being the wild-type CheYp concentration for which a steady - state invariant time ratio was obtained. Both the functional dependency and CheYp value are well documented in literature [2].
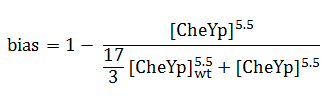
At every time point of the simulation, the cell probabilistically chooses whether to employ directed movement or to tumble, depending on the value of the bias (later described in the Algorithm subsection).
Mean times
An important way of characterizing the cell's behavior is by statistically observing it over a longer time period.
From this aspect, the mean tumbling length is the average time the cell spends in between two directed movement states, while the mean run length is the average time spent in the directed movement state.
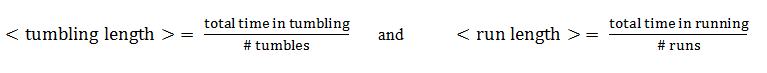
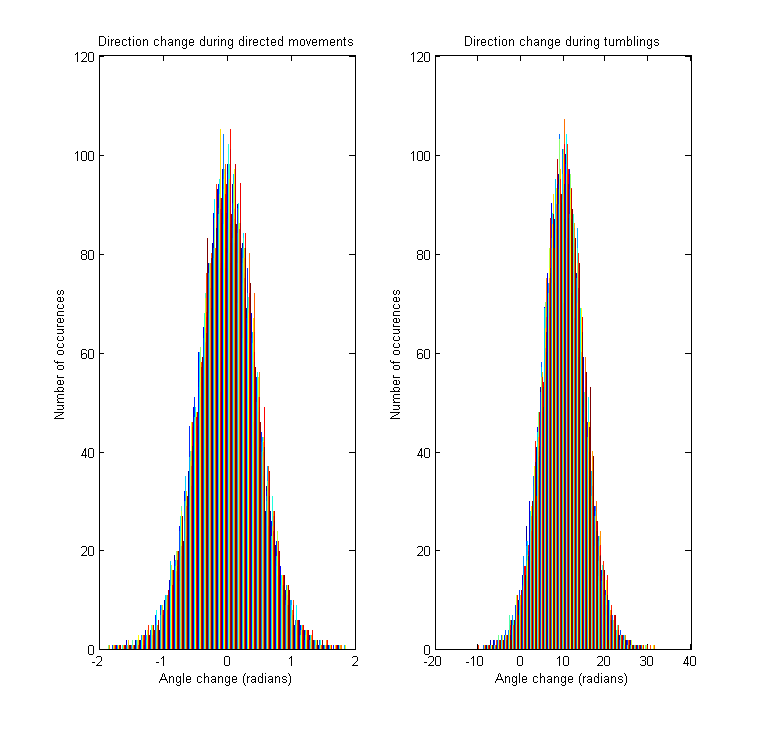
Direction changes
The tumbling angle is the change of direction between two directed movements. Since we are working with a continuous model, the derivative of the angle (i.e. angular velocity) is sampled for every tumbling, from a distribution resembling the empirically observed one [1].
As stated above, the model allows direction changes also during directed movements periods. In contrast to the tumbling state, this angle change is much smaller, being drawn from a normal distribution with 0 mean and standard deviation obtained from the literature [1].
Since the cell can move either clockwise or couterclockwise at every step of the simulation, the angle and tumbling speed distributions are always symmetrical about 0.
This ensures a realistic simulation of bacterial movement, not only asymptotically, analyzed through statistical estimates, but also in each time - point, analyzed through individual changes in spatial coordinates and direction.
Transition Probabilities
One of the biggest challenges regarding the movement model was obtaining a time - step - invariant behavior of the cell, whose reliability of statistical estimates should be the same, regardless how often the cell had to choose its future state.
In order to achieve this, we opted for a two - state first - order Markov process, in which the future state is only dependent on the current state. Since we have boolean values for any possible state, we can define the following four transition probabilities, separately controlled depending on whether the cell is currently in the directive movement or in the tumbling state.
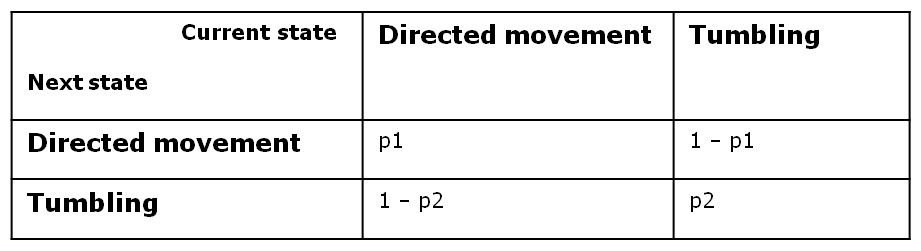
Therefore, the two central parameters of our model are the probabilities of keeping the current state also as future state: the probability that the future state is directed movement, when the current state is directed movement (p1) and probability of the future state being tumbling, when the current state is tumbling (p2).
In deriving the expression of these probabilities, we separately and symmetrically focused on the two processes: directed movement and tumbling. As a consequence, the mean run length is only dependent on the timestep and on the probability of continuing the directed movement (p1), while the mean tumbling length is only dependent on the timestep and on the probability of continuing tumbling (p2).
We will explain in detail the technique employed in deriving the probability of being in the directed movement state, when the previous state was directed movement. The symmetrical calculations, for the mean tumbling length, follow identically.
The mean run length is the expected value of a random variable representing the number of timesteps the cell consecutively spends in directed movement. By expanding the definition of an expected value, the mean run length becomes an infinite sum over all possible consecutive run-lengths, multiplied by their respective occurrence probabilities.
Assumptions
The assumptions of our model were taken in accordance with the existing experimental data on the chemotaxis movement [1]. The mean tumbling length was assumed to be constant, unlike the mean run length, which is a function of the bias. Besides the experimental evidence, this assumption also has a biological interpretation: tumbling is believed to occur because the flagella push each other away and disassemble after rotating counter-clock wise (directed movement). The duration spent in tumbling is a stochastic variable mainly representing the time the flagella afterwards need to reassemble, and a single process of reassembling is only weakly correlated with the frequency of tumbling.
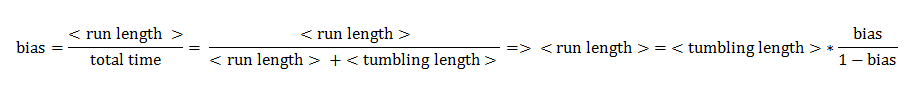
Furthermore we assumed that the mean velocity during directed movements is constant and that the angle change during one tumbling block is constant.
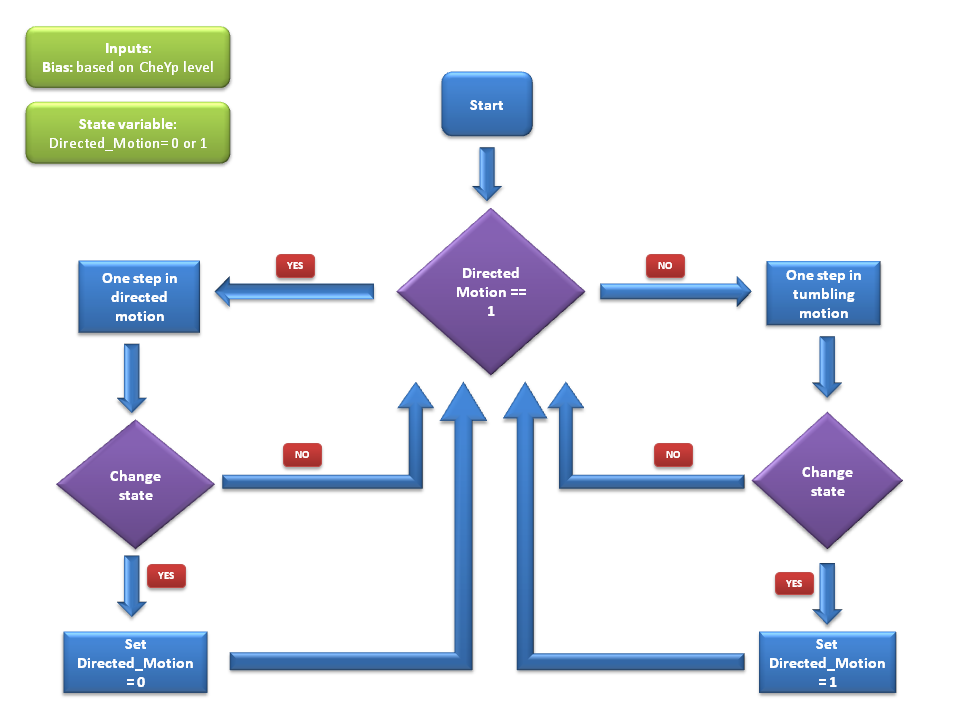
Algorithm
We use a two state model that reflects the two states the cell can be in through out its motion - swimming state & the tumbling state. At each time-step of the simulation, the cell will either stay in the same state or will switch the state according to these probabilities. Details of these probability values can be found below.
In each of these states, there is a transition probability to switch to the other states as described above. (We have the probability P1 that tells us the probability of switching to the tumbling state when the cell is currently in the swimming state and also the probability P2 that that reflects the probability of switching to the running state). At each timestep of the algorithm, the decision whether to run or tumble in the next timestep is taken based on these two probabilities.
If the decision was to run in the next timestep, the cell will be moved to it's next point with a velocity that agrees with literature [1]. Also, there is a slight deviation in the angle it moves during running. This angle is much smaller than the angle change during a tumbling timestep & it is sampled from a distribution that is suggested based on empirical data [1].
During a tumbling timestep, the angle of tumbling is decided such that the tumbling angle (between two directed motion segments) is a Weibull distributed random variable as suggested in [1].
We have made sure that the movement simulated using the algorithm agrees with the observed behaviour of E. coli in chemotaxis motion.
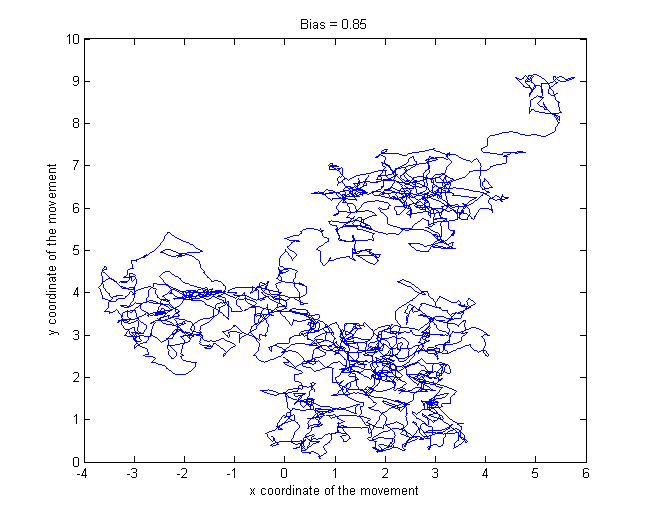
Simulation Results
The most interesting part regarding the results of the Movement Model is the comparison of the behavior of the bacterium at different values of the input bias. Figure 2 shows simulations lasting 2500 seconds, for different bias values (final image to be uploaded)
 "
"