Team:ETHZ Basel/Biology/Molecular Mechanism
From 2010.igem.org
Molecular Mechanism
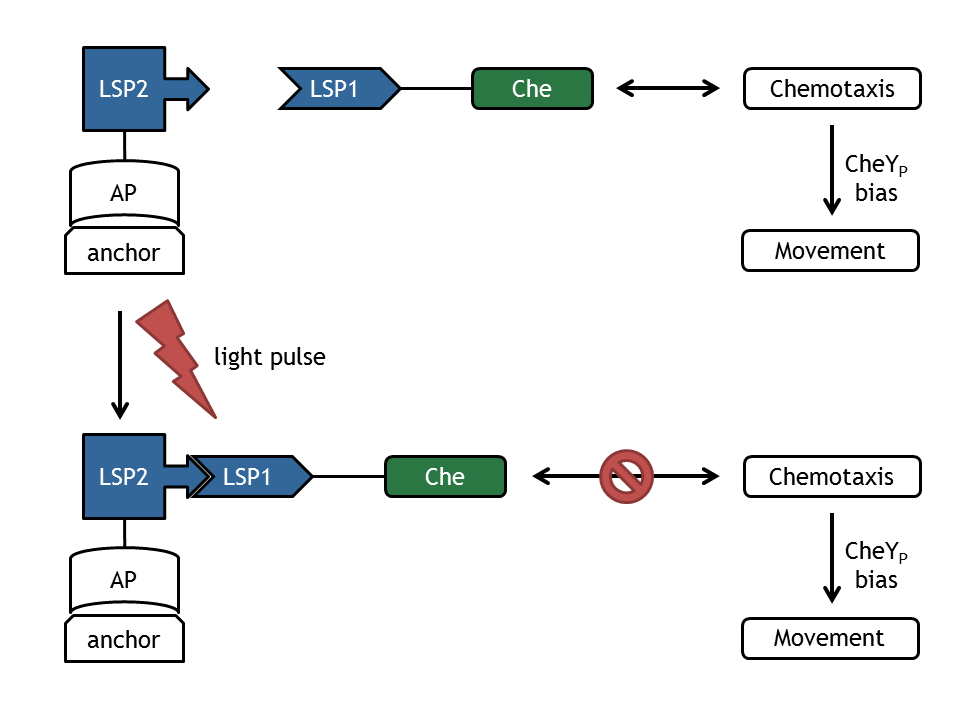
The core element of E. lemming is the photodimerization of the light-sensitive protein LSP to Che, which is a protein of the chemotaxis pathway. Upon change of wavelength of light pulses, this component will fuse with the corresponding light-sensitive protein (LSP'), which is linked to an anchor protein, bound to an anchor (plasmid). This results in the spatial relocalization of Che and in perturbation of the chemotaxis pathway. This process alters the ratio between tumbling and directed flagellar movement state.
Network topology
The chemotactic network is responsible for the directed movement of the bacterium, as a response to changes in the extracellular chemoattractant concentration. The two types of movement that the bacterium can employ are tumbling (i.e. change in angle and no change in position), occurring when the bacterium doesn’t sense an increase in attractant concentration and running straight ( i.e. change in position and no change in angle), occurring when the attractant concentration is sensed as increasing. The movement simulation algorithm (stochastic movement model) takes into consideration this change in the angle, as you can see here Movement. A running state is defined by BOTH a change in the movement angle AND a change in the spatial coordinates. As the E.coli runs along an axis, its coordinates obviously change. In contrast to this, during the tumbling state, only the movement angle changes. As the bacterium remains at its place, no change in the spatial coordinates is predicted. It is suggested by the model, that the stochastic distribution of the two states are CheYp-dependent. For example at CheYp = 12.5 [nM], both states are equally probable, but when CheYp is increased to 25 [nM], tumbling is dominant. Our models are strongly supported by other simulation studies, which predict that the phosphorylation level of CheY (which gives CheYp) directly influences the flagellar motor and therefore the dstochastic distribution of the two states. In E. coli, these two types of movements correspond to different rotation directions of its 4 flagellar motors (clockwise: tumbling; counter-clockwise: runs).
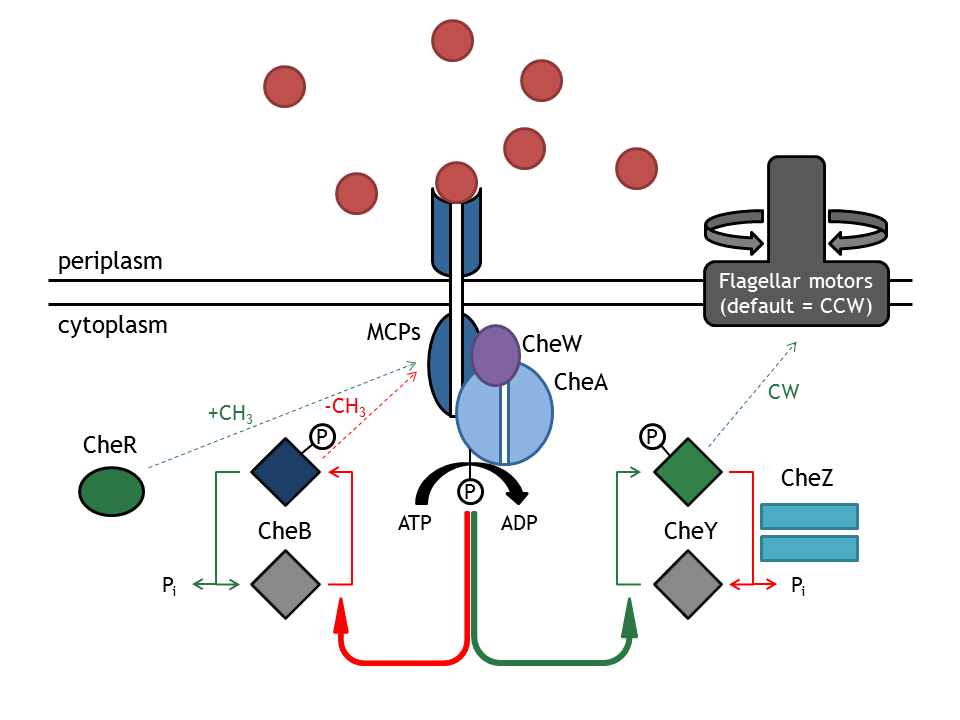
The signal transduction network consists of membrane receptor proteins (MCPs) and intracellular proteins (Che). MCPs sense the change in input concentration and communicate the information to the proteins CheW and CheA, located inside the cell. [0]
CheA autophosphorylation is the key process that determines whether the bacterium will run or tumble. This process is favored either by the increase in the methylation state of the membrane complex, done by CheR, or by decreasing attractant concentration.
Che-protein localization in the cell
The control of the tumbling frequency of E. coli is achieved by spatially localizing certain elements of the chemotactic network (Che proteins) and thus affecting the activity of their downstream partners. Inside the cell, the chemotactic proteins CheA, CheY and CheZ tend to co-localize with methyl accepting chemotaxis protein MCPs at the membrane. But whereas CheA and CheZ nearly only localize at the MCPs, CheY is also present in significant concentrations in the cytoplasm making it's localization more straightforward [1].
Anchor proteins for spatial localization
For spatial localization of the Che-protein complex, three different proteins will be utilized:
1. The tetracyclin repressor tetR anchoring the Che-protein to the DNA by binding to it's operator site tetO that has been inserted into a plasmid [2].
2. The triggor factor binding to the large ribosomal subunit [3].
3. The prokaryotic actin homologue MreB which assembles into helical filaments underneath the cytoplasmic membrane [4].
These anchors should enable to control the tumbling frequency by localizing a Che-protein and therefore interfering with its activity.
Light activated system: PhyB-Pif3
Properties of holophytochrome
In plants and some bacteria, members of the photoreceptor family of phytochromes regulate phototaxis, photosynthesis and production of protective pigments in response to light stimuli. The chromophore is encoded in the N-terminal domain of the protein [5]. There are two mayor types of phytochromes, type I (PhyA) and type II (PhyB and PhyC) [6, p.142]. Both exist in a biologically inactive form Pr absorbing red light and an active configuration Pfr which absorbs far-red light using a covalently attached tetraphyrrole chromophore for light absorption [5]. The interconversion between these two states takes only miliseconds [7]. While the Pr form is very stable (half live of about 100h), the Pfr form is faster degraded (type I half-life between 30min and 2h, type II half live around 8h) [6, p.142 ff].
Light-switchable gene systems utilize the phytochrome interacting factor Pif3, a basic helix-loop-helix protein, for light-induced gene expression. The N-Terminus of Pif3 selectively binds the Prf form of the phytochrome and rapidely dissociates in response to reconversion to the Pr state [7].
For the design of a light-activated system in Bacteria one has to consider that chromophores such as phycocyanobilin PBS are not naturally present; therefore, they either have to be added to the media or two genes for its biosynthesis starting from Haem have to be introduced [8]. Furthermore, the fact that phyotochromes can form sequesters in the Prf state in the timescale of seconds has to be examined [6].
References
[0] M.J. Tindall, S.L. Porter, P.K. Maini, G. Gaglia, J.P. Armitage. Bulletin of Mathematical Biology (2008) 70: 1525–1569
[1] Sourjik and Berg: Localization of components of the chemotaxis machinery of Escheria coli using fluorescent protein fusions. Molecular Biology. 2000; 37:4.
[2] Bertram and HIllen: The application of Tet repressor in prokaryotic gene regulation and expression. Microbial Biotechnology. 2008; 1:1.
[3] Hesterkamp, Deuerling and Bukau: The Amino-terminal 118 amino acids of Escherichia coli Trigger factor constitute a domain that is necessary and sufficient for binding to ribosomes. The Journal of Biologcial Chemistry. 1997; 272:35.
[4] Kruse, Bork-Jensen and Gerdes: The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Molecular Microbiology. 2005;55:1.
[5] Sato, Hug, Tepperman and Quail: A light-switchable gene promoter system. Nature Biotechnology. 2002; 20.
[6] Kendrick and Kronenberg: Photomorphogenesis in plants. Kluwer academic publishers, Dordrecht, The Netherlands. 2nd edition, 1994.
[7] Keyes and Mills: Inducible systems see light. Trends in Biotechnology. 2003; 21:2.
[8] Levskaya, Chevalier, Tabor, Simpson, Lavery, Levy, Davidson, Scourast, Ellington, Marcotte and Voigt: Engineering Escherichia coli to see light. Nature Brief Communications. 2005, 438.
[9] Amy B Tyszkiewicz and Tom W Muir: Activation of protein splicing with light in yeast. Nature Methods Brief Communications. 2008, 5:303-305
[10] Yuri Matsuzakia, Shinichi Kikuchia and Masaru Tomita: Robust effects of Tsr–CheBp and CheA–CheYp affinity in bacterial chemotaxis. Artificial Intelligence in Medicine. 2007; 41:2
Laboratory of Synthetic Protein Chemistry, The Rockefeller University, 1230 York Ave., New York, New York 10065, USA.
 "
"