Team:ETHZ Basel/Biology/Implementation
From 2010.igem.org
Implementation
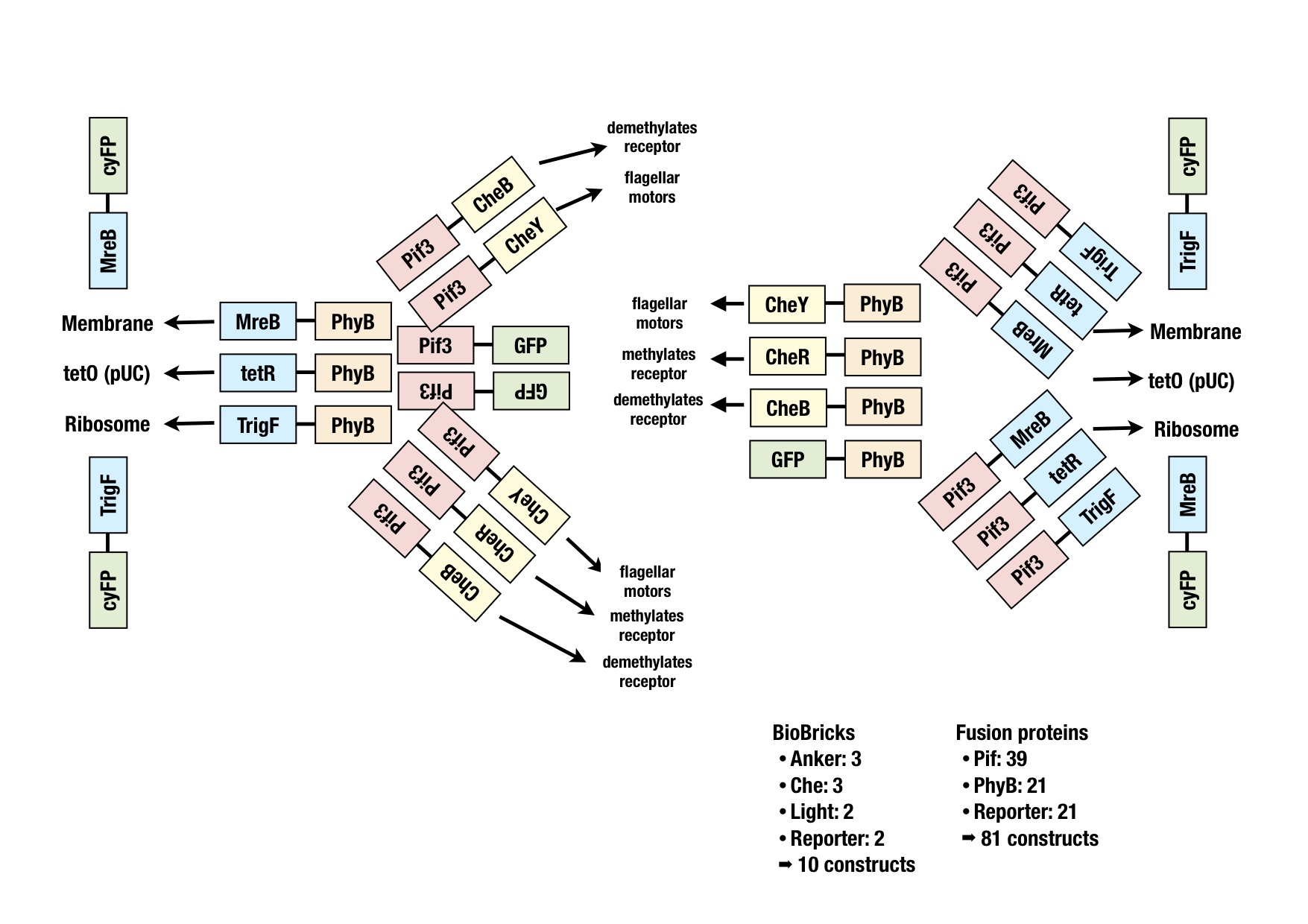
Generation of fusion proteins
The picture graphically represents all the fusion proteins we have in our wetlab production pipeline. The BioBricks for all those constructs were generated either via PCR or synthesized from GeneArt. For sequencing, the bricks were then ligated into the storage vector. Cutting and pasting of constructs into working vectors should be feasible, applying the cloning strategy BBF RFC28 [1].
Experimental Design
Due to the high amount of fusion proteins which were in planing (81 constructs), priorities had to be distributed to the different genes. This was possible due to the various models the dry-lab team implemented.
- Chemotaxis protein: CheY was chosen as first target.
- Anchor: TetR was the first choice due to its wide application in synthetic biology and extensive characterization.
- Pif3 linked to Che-Protein: Because PhyB has been claimed to have a tendency to sequester in plants, it was chosen not to be linked to the Che protein.
- Ratio anchor to binding partner: The simulations favoured a ratio of 50 µM anchor to 40 µM anchor binding partner.
Plasmid copy number
As the ratio between the amount of anchor and its binding partner has been shown to be essential, according to the experimental design evaluation, the plasmid copy number was determined by the normalization of cell number via optical density measurement followed by plasmid concentration measurements using a commercial Miniprep kit.
From a modeling perspective, working vector 1 (BBR1 ori) should have a higher copy number than working vector 2 (RK2 ori). The results show that working vector 1 has a 1.1x higher frequency in the cell that working vector 2. This is acceptable, as molecular modeling suggests an optimal ratio of 1.5x.
In view of the proportion of anchor to anchor binding protein, the aim of tetO7 = 50 µM in one cell can not even be achieved by ligation into a high copy number plasmid such as pUC19. The measured amount of 266 vectors in one cell gives approximately 5 µM of tetO binding sites. Therefore, the decision was made to integrate a second anchor binding protein which is also fused to the light sensitive protein PhyB in one operon.
Functionality assays
The constructs are tested for the following properties:
- Che protein fusion: Using the chemotactic assay described by Mazumder et al. [2], the functionality of Che protein fusions can be tested.
- Localizer fusion: Spatial localization of the anchor protein to either the plasmid (tetR-tetO), the cell membrane (mreB) or the ribosome (trigA) can be investigated by fusing the anchor binding protein to a fluorescent protein.
- PhyB-Pif3 system: Fusing a second fluorescent protein to Pif3 would enable the visualization of the light activated dimerization.
References
[1] BBF RFC 28: A method for combinatorial multi-part assembly based on the Type IIs restriction enzyme AarI. Peisajovich et al. (2009)
[2] Determining chemotactic responses by two subsurface microaerophiles using a simplified capillary assay method. Mazumder et al. (1999)
 "
"