Team:ETHZ Basel/Biology/Implementation
From 2010.igem.org
(→The problem of anchoring places - or how can we be sure, that all anchor-fusions find a place to anchor?) |
(→Experimental realization) |
||
| Line 13: | Line 13: | ||
== Experimental realization == | == Experimental realization == | ||
| + | <html><div class="thumb tright"><div class="thumbinner" style="width:482px;"> | ||
| + | <iframe title="YouTube video player" class="youtube-player" type="text/html" width="480" height="390" src="http://www.youtube.com/embed/qlUQpivONHc?rel=0&hd=1" frameborder="0"></iframe> | ||
| + | <div class="thumbcaption"><div class="magnify"><a href="http://www.youtube.com/watch?v=qlUQpivONHc&hd=1" class="external" title="Enlarge"><img src="/wiki/skins/common/images/magnify-clip.png" width="15" height="11" alt="" /></a></div><b>Example movie demonstrating the initial problems we faced when imaging the first cells in the channel:</b> high passive flow, dirt in the microscope apparatus, and that the majority of the cells did not show any swimming behavior at all.</div></div></div></html> | ||
After getting the input from the experimental design we had to realize or verify the stated parameters in our biological implementation. | After getting the input from the experimental design we had to realize or verify the stated parameters in our biological implementation. | ||
Revision as of 08:59, 27 October 2010
Biological Implementation
Experimental design
Considering the tremendously hight amount of 81 fusion proteins, we had to assign priorities to the different possibilities. This was made with the various models the dry-lab team implemented in order to help us prioritizing. We had to consider the following parameters. The detailed explanations for our choices you find by clicking on the question itself which is internally linked to the appropriate wiki page:
- Which Che protein should we choose? - We chose CheY as the first target.
- Which anchor would work the best?: TetR was the first choice due to its wide application in synthetic biology and extensive characterization. Our second choice was the ribosome binding domain of trigA
- Which LSP should be fused to the Che protein. PhyB or Pif3?: There was no rational preference which LSP to fuse to the Che protein, so we decided to fuse PhyB to the Che protein.
- Ratio anchor to binding partner: The simulations favored a ratio of 50 µM anchor to 40 µM of anchor binding partner.
Experimental realization
Fusion proteins
First at all, according to the given input, we decided to assemble the following fusion proteins: CheY fused to PhyB as Che-fusion as well as TetR fused to Pif3 and Trigger factor fused to Pif3 as anchor-fusions.
Implementation chassis
As we want to change the tumbling frequency of E. lemming by spatial localization of CheY it is important to use a strain for implementation which does not express wild type CheY but only our CheY fusion protein. Therefore, the system will be implemented in a cheY knock out strain which was taken from the KEIO collection. For implementing E. lemming using trigger factor as anchor, we constructed a double knock out tig cheY as all ribosomes should be available to bind the trigger factor fusion protein and not WT trigger factor. This strain was constructed by P1 phage transduction using both strains from the KEIO collection.
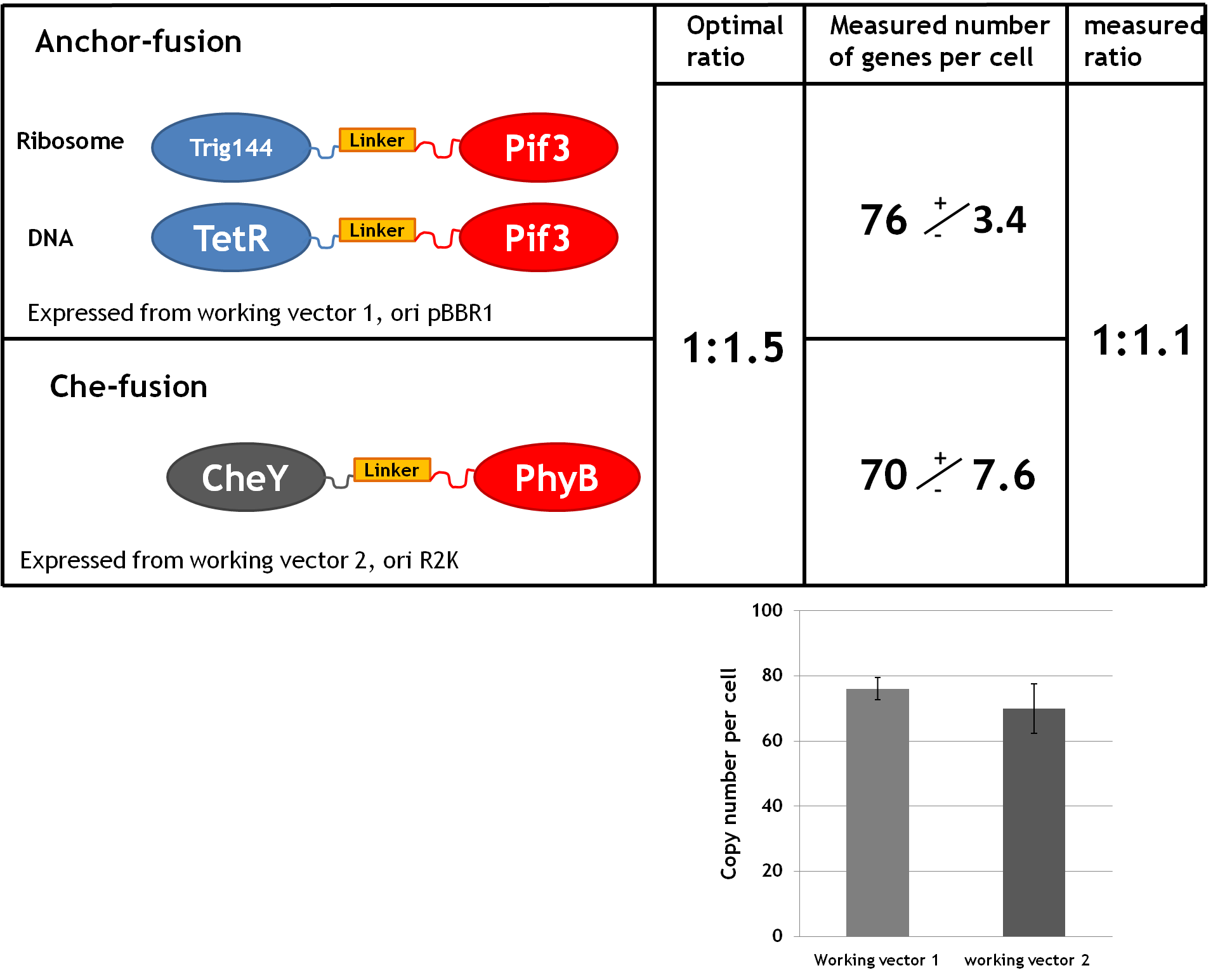
Ratio between anchor-fusion and CheY-fusion
For the implementation of E. lemming we need to express two fusion proteins simultaneously in one cell. The LSP1 fused to the anchor and the LSP2 fused to the Che protein. To ensure that all Che proteins can be localized, the anchor fusion proteins should be present in slightly higher amounts. The experimental design favored a ration of 1.5 (50 uM of anchor fusion to 40 uM of Che-fusion). To keep the system as simple as possible we express both proteins from the same type of promotor, the arabinose inducable PBAD promotor. Thus, the only way to adjust the amount of fusion protein expression was by the number of gene copies per cell. We therefore constructed the working vectors based on two different origins of replication (pBB1 and RK2) and measured the actual copy number per cell to verify the optimal ratio of 1.5. The plasmid copy number was determined by the normalization of cell number via optical density measurement followed by plasmid concentration measurements (using a commercial Miniprep kit). The results are shown on the right.
The problem of anchoring places - or how can we be sure, that all anchor-fusions find a place to anchor?
As mentioned above, we chose to implement TetR and trigger factor as anchors for the localization of CheY. For successful anchoring we needed to be sure to provide enough anchor places within one cell for the anchor to bind to. For TetR that means that we have to provide enough tet operator sites where it can bind to. For Trigger factor we need enough ribosomes it can bind to. In both cases this means that we have to provide about 50 µM of anchor places.
Can we provide enough anchor places for TetR? 7 Tet operator sites (sites were TetR bind to on the DNA) were cloned into pUC19 (ori ColE1) and the natural ampicillin resistance of pUC19 was changed to a gentamycin resistance to make it compatible to our working vectors which express an ampicillin resistance and a spectinomycin resistance. To calculate the amount of tet operator sites per cell we determined the copy number of pUC19 and calculated the estimated anchor places. As TetR binds as a dimer to each operator site, 14 TetR can anchor on one plasmid. As we measured to have 267 copies of pUC19 per cells we can provide anchor places per cell. Assuming a cell size of 1 µm this gives about 2.7 µM of anchor places. The results of our calculation are shown on the right.
Can we provide enough anchor sites for trigger factor?
Regarding Bionumbers (The Database for useful biological numbers) the number of ribosomes per cell strongly depends on the growth phase.
In view of the proportion of anchor to anchor binding protein, the aim of an intracellular tetO7 concentration of 50 µM can't be achieved, even not by ligation of the tet07 construct into a high copy number plasmid such as pUC19. The measured amount of 266 vectors per cell results in the exposure of approximately 5 µM of tetO binding sites. Therefore, we decided to integrate a second anchor binding protein into the plasmid, which is also fused to the light sensitive protein PhyB in one operon.
Functionality assays
The constructs are tested for the following properties:
- Che protein fusion: Using the chemotaxis assay described by Mazumder et al. [2], the functionality of Che protein fusions can be tested.
- Localizer fusion: The spatial localization of the anchor protein can be investigated by fusing it to a fluorescent protein (fluorescent GFP-tag). The anchor protein can fuse to the plasmid (tetR-tetO), the cell membrane (MreB) or the ribosome (TrigA).
- PhyB-Pif3 system: Fusing a second fluorescent protein to Pif3 would enable us to visualize of the light-dimerization (photodimerization).
References
[1] BBF RFC 28: A method for combinatorial multi-part assembly based on the Type IIs restriction enzyme AarI. Peisajovich et al. (2009)
[2] Determining chemotactic responses by two subsurface microaerophiles using a simplified capillary assay method. Mazumder et al. (1999)
 "
"