Team:ETHZ Basel/Biology/Cloning
From 2010.igem.org
Cloning strategy for the construction of our Biobricks
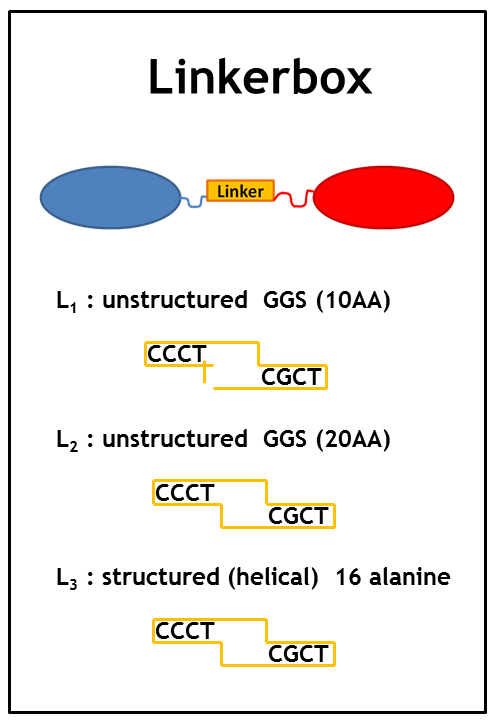
As we planned to generate several fusion proteins with different linkers, we decided to use the cloning strategy BBF RFC28: A method for combinatorial multi-part assembly based on the Type IIs restriction enzyme AarI [1]. The advantage of this strategy is that we can -simultaneously-clone up to 3 different inserts into one expression vector! In the following section we give an overview of how this was achieved.
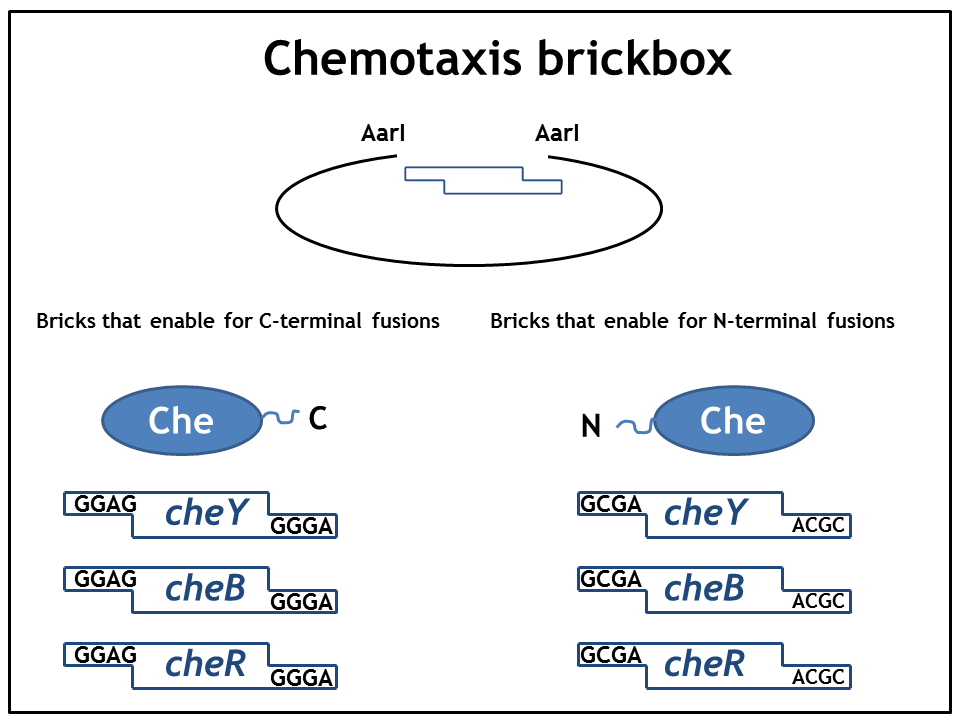
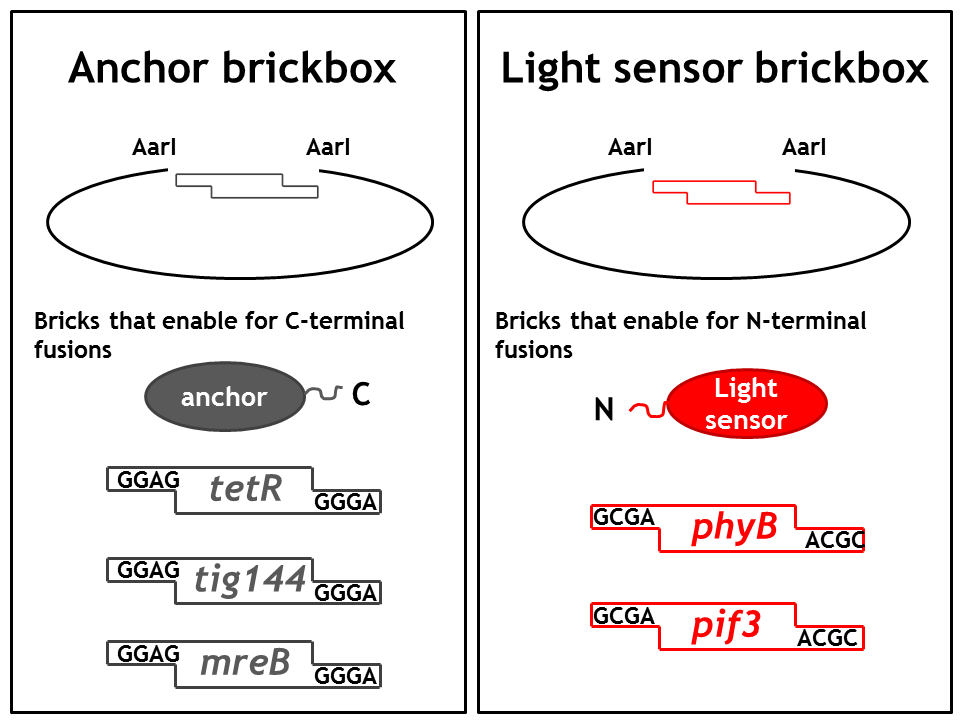
1. Step: Construction of brickboxes which enable the generation of fusion proteins
Those Parts were generated by PCR. We used primers which were specified in the BBF RFC28 manual and subcloned into the storage vector pSEVA132 (Victor de Lorenzo's lab, KanR, pBBR1 ori) by blunt end ligation. The plasmid pSEVA132 enables us to pre-screen the transformation products by blue white screening. This making the generation of the brickbox parts very efficient. Generated parts were verified by AarI digest and sequencing. Due to the occurence of rare codons in the sequence of PhyB and Pif3, these two genes were codon-optimized (ordered from GeneArt). As the implementation of E. Lemming relies on two protein fusions (the "anchor-light sensor" and "light sensor-che protein" fusion), we constructed two expression vectors (we call them working vectors), which enable us to simultaneously express the two fusion proteins in the same plasmid.
- Working vector 1 is a derivative of pSEVA132 conferring resistance to ampicillin and replicating with ori pBB1.
- Working vector 2 is a derivative of pSEVA421 expressing a spectinomycin resistance cassette and replicating with ori RK2. The gene for the repressor AraC and the corresponding ParaBAD promotor/operator sites were introduced into both vectors followed by an insert, which is flanked by AarI-recognition sites. Digest with AarI releases this insert and generates a vector with assembly-compatible overhangs.
2. Step: Assembly of fusion proteins using the generated brickboxes
The following image illustrates the assembly of a fusion protein. In the section "Implementation" the constructed fusion proteins, required for the implementation of the synthetic network, are described. But how should the fusion process look like? Should we use the C-term or the A-term? Would this have an effect on the functionality of the biobricks? The decision which fusion strategy to choose, could be taken thanks to some models. The modeling group successfully tackled this predominant question and achieved to "in silico" formulate the crucial system parameters required for a working system.
Linking BBF RFC28 to the Tom Knight's original assembly standard (OAS):
General scheme for the design and construction of Tom Knight's OAS-compatible fusion proteins
BBF RFC28 is a method for combinatorial multi-part assembly based on the Type IIs restriction enzyme AarI and is a very efficient method to construct fusion proteins. As we have a great number of constructs to generate, efficiency in the cloning process is vital for the project. Unfortunately it is not compatible with the general iGEM standardized biobricks. As a consequence, Tom Knight's original assembly standard is not compatible with those fusion proteins, as they don't contain the required prefix and suffix sequences. During our project we learned a lot about the cloning of fusion proteins using BBF RFC28-especially how to make it work efficiently. We would like to share our experience here, especially as we worked out a general design method for the easy construction of Tom Knight's OAS and BBF RFC28 compatible working vectors (the expression vector that is finally used for the assembly) that can be used for the final assembly step. Our vector design allows in addition for a GFP (or any other reporter gene)-mediated screening for fusion protein containing clones.
As we had already started the cloning procedure before learning about the little difficulties of the assembly method and before making up our minds how to improve it and actually link it to the Tom Knight's BBF, our fusion proteins were not cloned into a Tom Knight's OAS-compatible working vector. Nevertheless, the design scheme that is outlined below is under construction.
In general the working vector needs to contain an insert flanked by AarI-sites. These cleavage sites need to be designed such that digestion with AarI releases the insert and leaves a vector backbone with 5` overhangs compatible with the BBF RFC 28 standard. To isolate 100% cleaved vector backbone for efficient assembly, Insert, cleaved vector and uncut vector can be separated on a 1% agarose gel. This is crucial as AarI does not cleave every cleavage site with high efficiency and alarge fraction will be uncut vector. By choosing a constitutively expressed GFP (it can be any other constitutively expressed reporter gene) as insert, positive clones containing the assembled protein can be distinguished from clones containing the original insert (traces of uncut vector might still be present in the cleaved vector fraction) by green/white screening. Constitutive expression of the reporter is convenient as no inducer needs to be added to the selection plates givign total freedom of choice for the fusion protein expression system. An existing biobrick - the GFP generator XXXXX - has therefore been investigated and characterized. When located on a high copy vector GFP is expressed in high amounts even in the absence of inducer (IPTG) although its promotor containes a lac operator site.
 "
"