Team:ETHZ Basel/Achievements/E lemming
From 2010.igem.org
The E. lemming
What it needs to bring E. lemming alive
It needs an archeal photoreceptor that is fused to a bacterial chemotactic transducer. This was successfully demonstrated by Jung et al. in 2001, who fused the Natronobacterium pharaonis NpSRII (Np seven-transmembrane retinylidene photoreceptor sensory rhodopsins II) and their cognate transducer HtrII to the cytoplasmic domain of the chemotaxis transducer EcTsr of Escherichia coli. For more information visit our Archeal Light Receptor wiki page.
To make the nice videos shown below, the optimal chemotactic conditions, that were concluded from a series of different microscopy images, were applied. Escherichia coli K12 cells were grown at 30 °C in Lysogeny Broth to on OD of 1.0. IPTG for induction of gene expression and all-trans retinal for NpSRII folding were added to the media.
Experimental Results
We imaged several transfected E. coli cells with a 20× lens in a ≈50μm high flow channel. Approximately 5% of the cells reacted on the switch-on and -off of the blue light signal by changing significantly their swimming behavior. In Video 1 shows an E. lemming swimming in regular circles in a constant light environment. When switching the blue light on, it completely changes its motility after a 2-3s delay by swimming straight for several seconds. When the light is switched off, it returns to its original behavior after a similar delay (see paragraph "Characterization").
Video 2 shows another E. lemming which is swimming straight with frequent interruptions by tumblings when being in a constant light environment. When the blue light is switched on, the tumblings nearly completely disappear and the E. lemming is swimming straight over large distances. When the light is switched off, the tumbling disappears or the E. lemming alternatively stops movement at all.
|
Video 1: This video shows the E. lemming in action. The unprocessed microscope images are available here. |
Video 2: this the brother of the E. lemming, who decided to swim several times nearly out of focus and out of the field of view such that he had to be tracked manually.. The unprocessed microscope images are available here. |
In both movies we visually highlighted the current position of the respective E. lemming. In the first movie this was possible by using our cell detection and tracking algorithm, such that also all other cells could be easily highlighted, too. In the second video this was not possible, since the E. lemming nearly swims out-of-focus once and the stage had to be moved during the experiment to keep the E. lemming in the field of view of the microscope. Thus, the tracking had to be done by hand (≈230 frames) for the second movie.
Characterization
To characterize the change of swimming behavior when switching on or off the blue light signal, we estimated the angle of the E. lemming for each frame of Video 1. This was done by obtaining the positions (xi, yi) of the E. lemming from our cell detection and tracking algorithm. The angle φi of frame i was then calculated by central differences:
tan(φi)=(yi+1-yi-1)/(xi+1-xi-1).
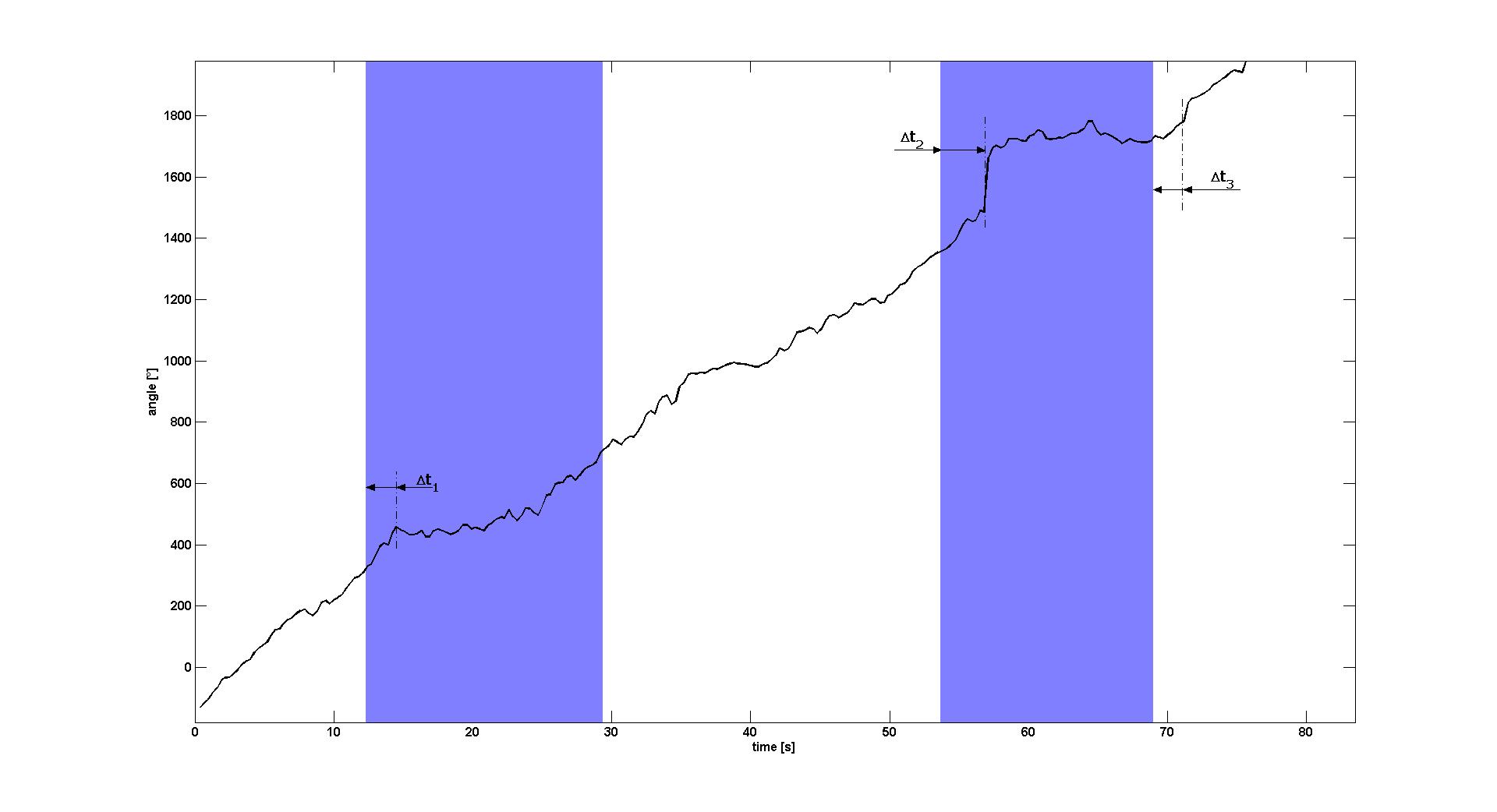
 "
"


