Team:EPF Lausanne/Project parts
From 2010.igem.org


Cloning
We performed all the cloning in E.coli, and did experiments on Asaia transformation, growth and resistance to antibiotics in parallel.
As E. coli origin does not work in Asaia, we added an origin that was compatible with this bacteria (which was recovered from a GFP-plasmid transformed into the Asaia we received from Italy). On the opposite, Asaia's origin does work in E. coli, which is very useful for our sets of experiments.
BioBricks
All our parts are in the pSB1C3 backbone vector (abbreviate C3). When you click on the part's name, you are redirected to the partsregistry corresponding page.
First the legend:
- Ori = Origin of replication for Asaia and E. coli
- S = Strong Promoter
- W = Weak Promoter
- Immu = immunotoxin
- P25 = Pfs25 protein
- P28 = Pfs28 protein
- Kan = Resistance to Kanamycin
- Tet = Resistance to Tetracycline
We made basic parts to build the complexest one.
|
|
|
|

|

|

|

|
*C3 + Immu The immunotoxin is the main part of the project, we obtained it by synthetization. For a better understanding, go there.
*C3 + S We made a new strong promoter permitting use to test the expression of the immunotoxine. We called it strong because we used the constitutive sequence which should give the maximum level of expression.
*C3 + W This promoter has been made to have a second level of expression for our proteins, which is lower than the strong one.
We made the following BioBricks from the aboves :
You can see an overview of the all parts.

Promoter characterization
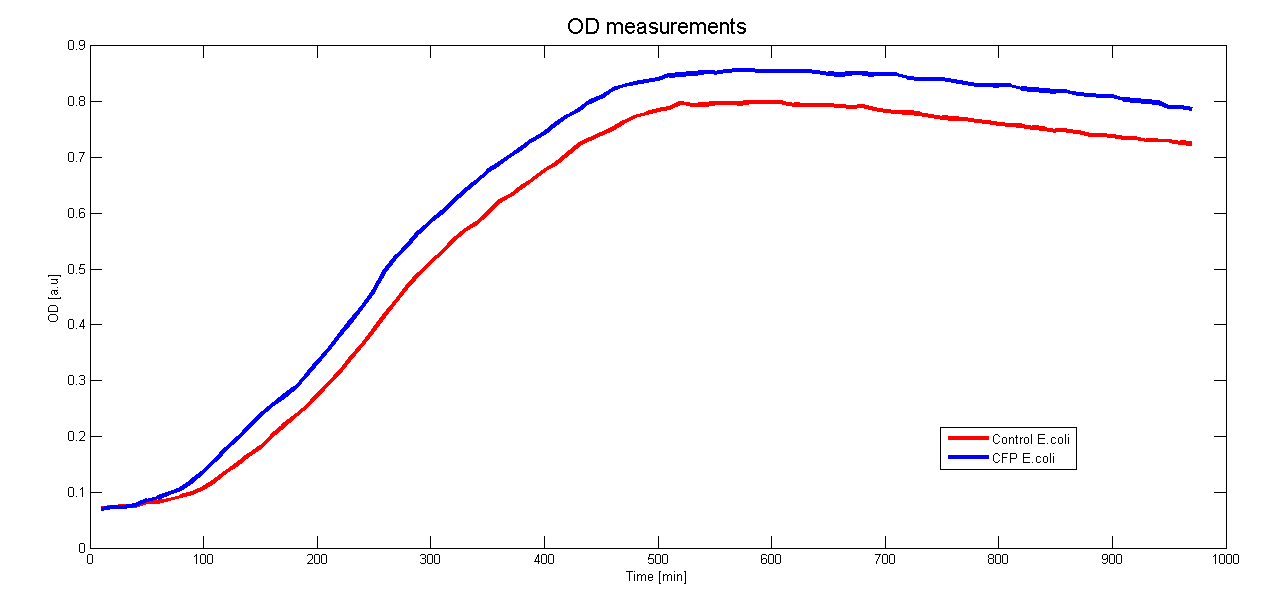
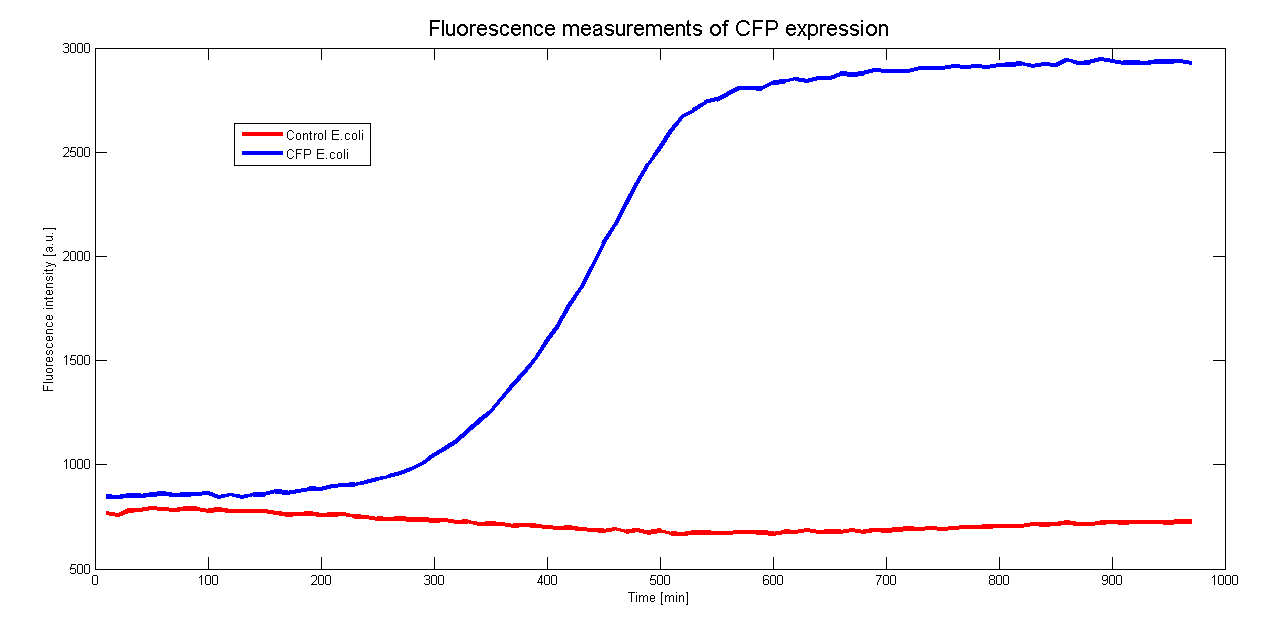
To characterize the promoter we want to use in Asaia we cloned it before a CFP gene. By monitoring the CFP expression in time we could measure the activity of the promoter. Moreover to see if the presence of the CFP hindrate the growth of the clones we measured the growth curve.
Both measurements were done contemporaneously in E.coli DH5a over 970 minutes (16 hours) at 37°C and the results are averaged over 6 samples.
The control for the autofluorescence and the growthcurve were done with a wildtype strain of DH5a.
Unfortunately we can't compare our measurements with a well known promoter.
Further characterisation will focus on the measurement of the promoter's activity in Asaia.

 "
"