Team:EPF Lausanne/Project parts
From 2010.igem.org
(→BioBricks) |
(→BioBricks) |
||
| Line 50: | Line 50: | ||
[[Image:Second three.png|870px]] | [[Image:Second three.png|870px]] | ||
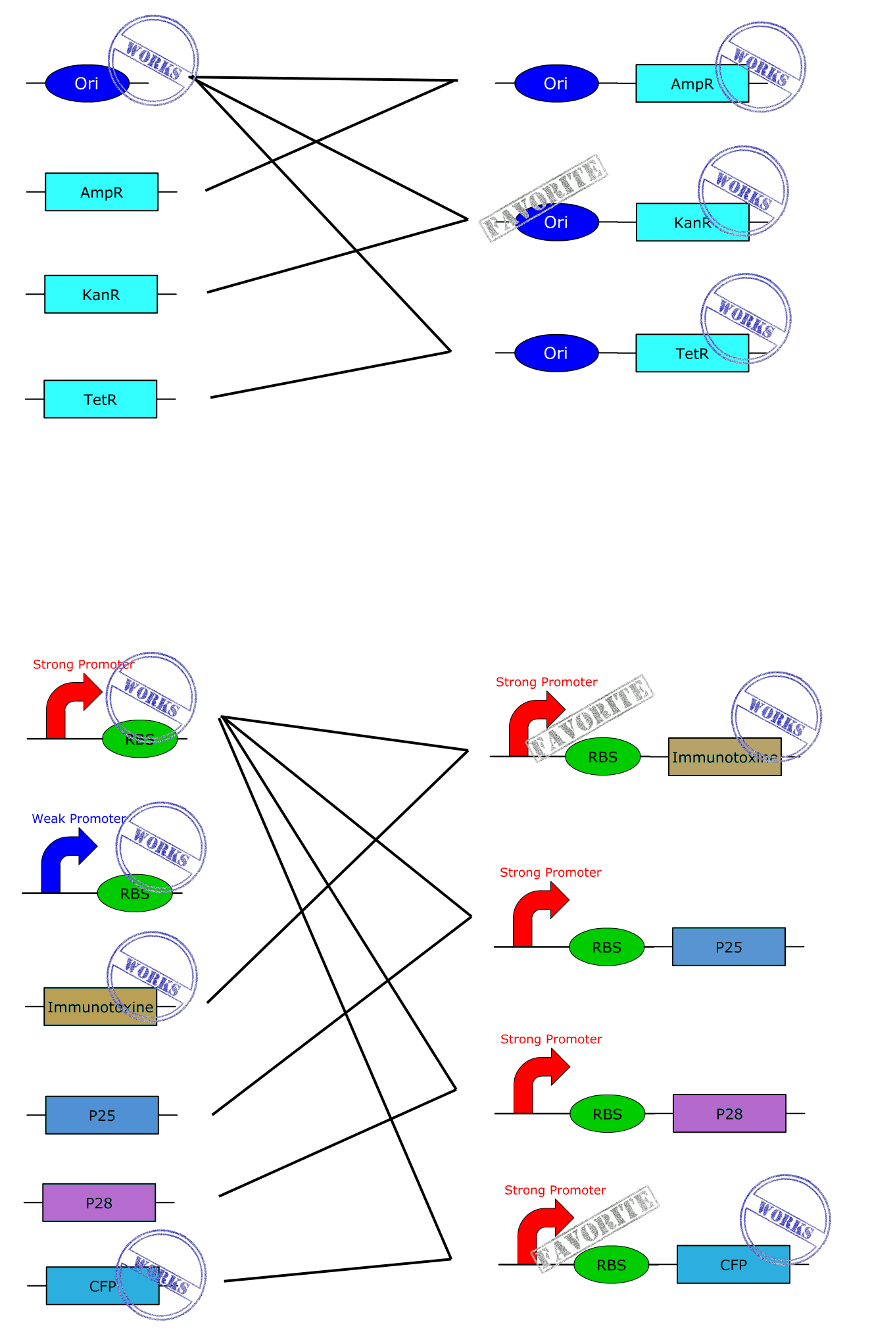
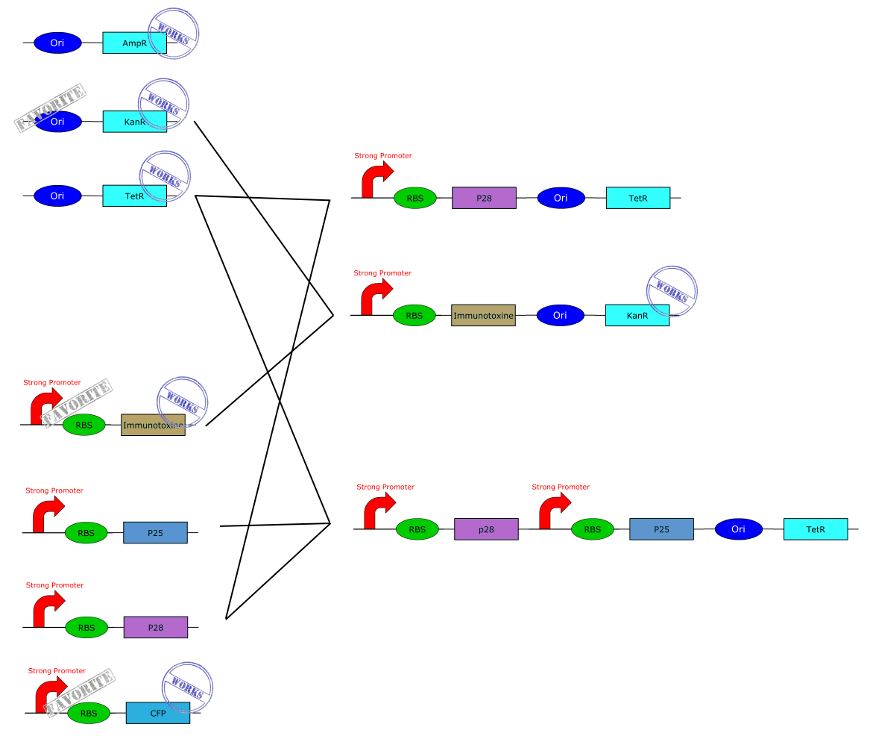
| - | + | Here you get a global overview of our BioBricks construction plan. | |
[[Image:EPFL_Three-petit.png|870px]] | [[Image:EPFL_Three-petit.png|870px]] | ||
Revision as of 10:28, 27 October 2010


Cloning
We performed all the cloning in E.coli, and did experiments on Asaia transformation, growth and resistance to antibiotics in parallel.
As E. coli origin does not work in Asaia, we added an origin that was compatible with this bacteria (which was recovered from a GFP-plasmid transformed into the Asaia we received from Italy). On the opposite, Asaia's origin does work in E. coli, which is very useful for our sets of experiments.
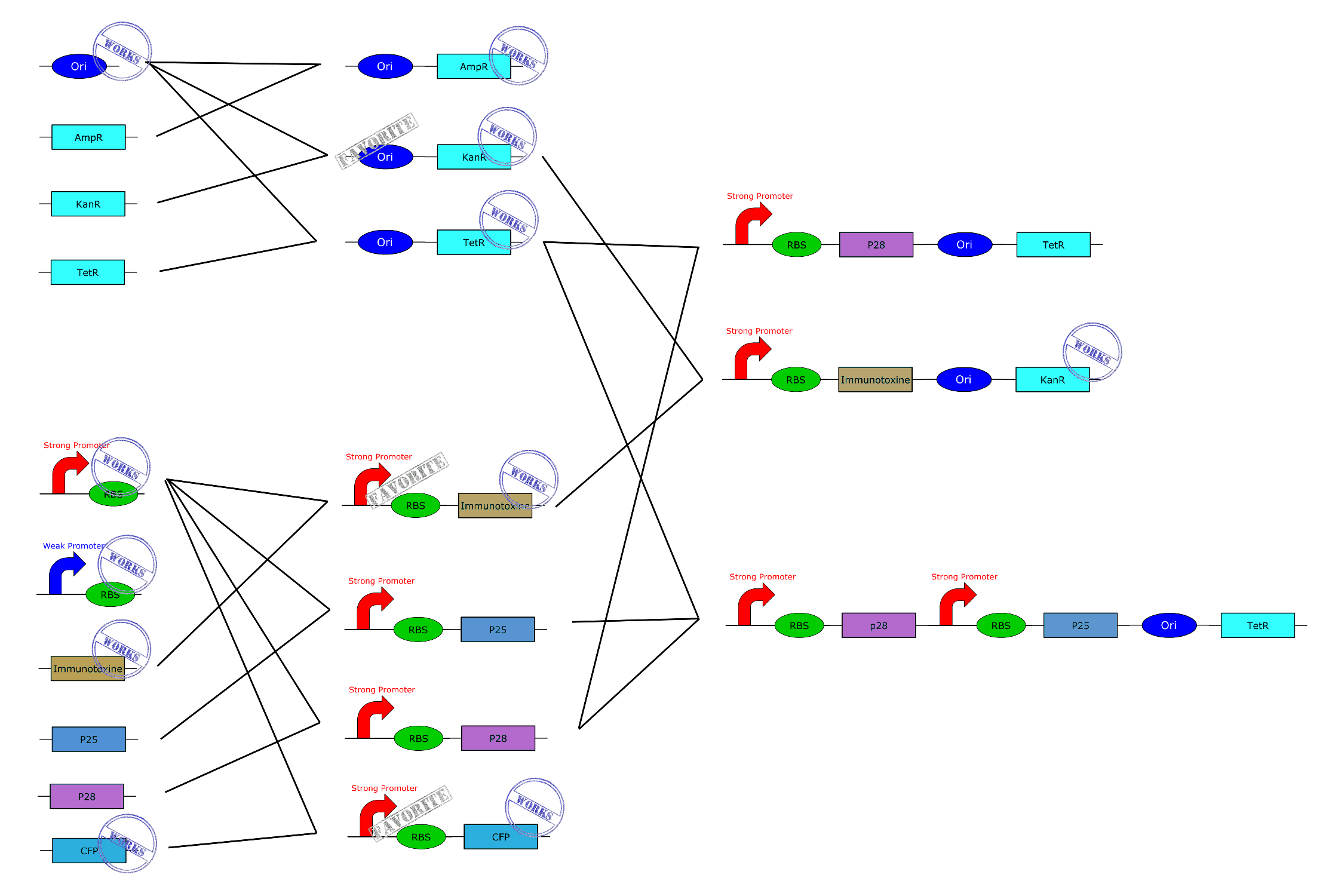
BioBricks
All our parts are in the pSB1C3 backbone vector (abbreviate C3).
First the legend:
- Ori = Origin of replication for Asaia and E. coli
- S = Strong Promoter
- W = Weak Promoter
- Immu = immunotoxin
- P25 = Pfs25 protein
- P28 = Pfs28 protein
- Kan = Resistance to Kanamycin
- Tet = Resistance to Tetracycline
This are the first and basics constructions we made. Just clic on the part to get more information.(Note de Christian, je vais faire les lien sur l'image cet après midi, après vos remarques car je l'ai déja fais 3 fois et du recommencer à zéra à cause des changement)
This are the BioBricks we obtain by using the one above.
Here you get a global overview of our BioBricks construction plan.
Promoter characterization
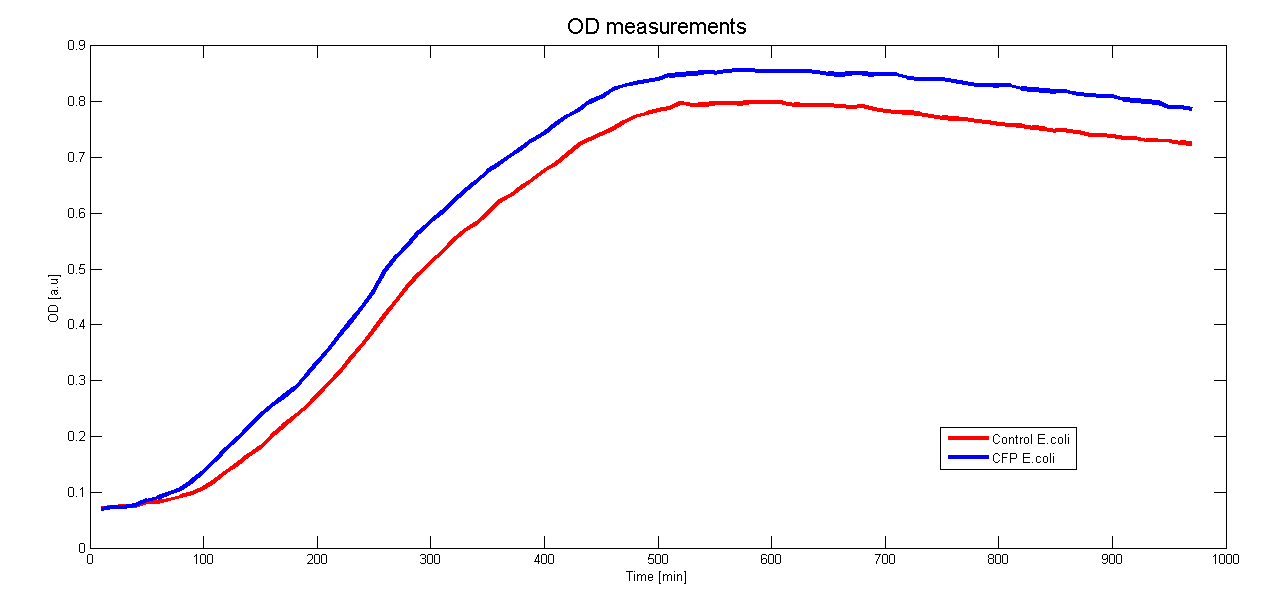
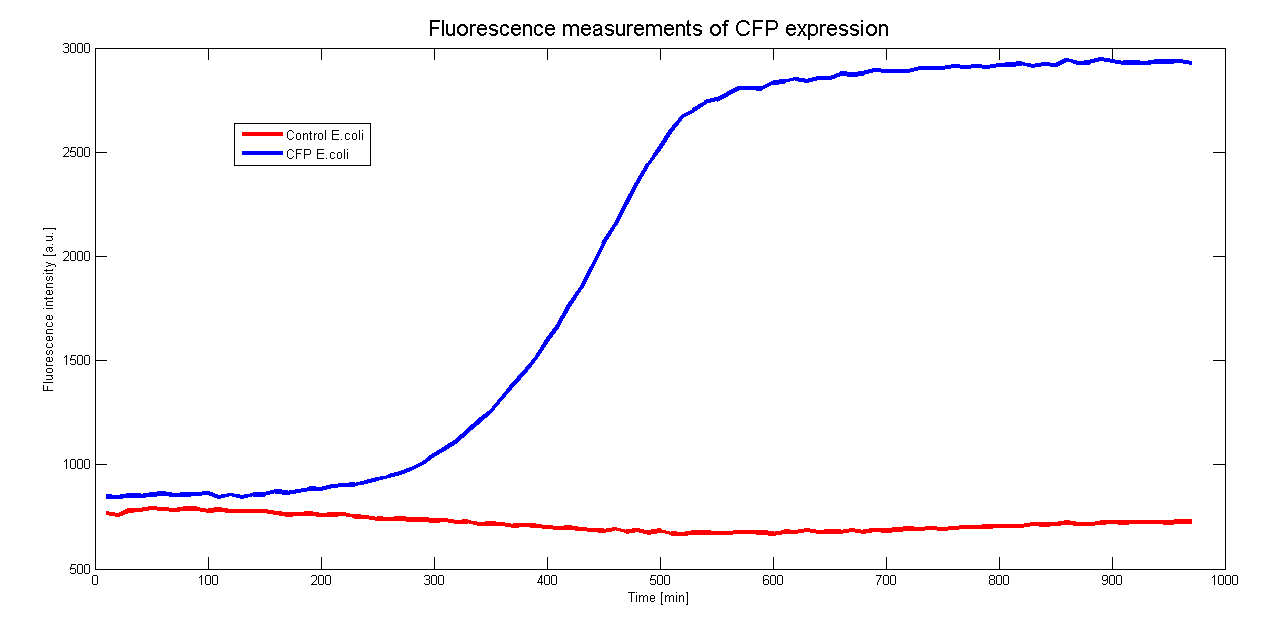
To characterize the promoter we want to use in Asaia we cloned it before a CFP gene. By monitoring the CFP expression in time we could measure the activity of the promoter. Moreover to see if the presence of the CFP hindrate the growth of the clones we measured the growth curve.
Both measurements were done contemporaneously in E.coli DH5a over 970 minutes (16 hours) at 37°C and the results are averaged over 6 samples.
The control for the autofluorescence and the growthcurve were done with a wildtype strain of DH5a.
Unfortunately we can't compare our measurements with a well known promoter.
Further characterisation will focus on the measurement of the promoter's activity in Asaia.

 "
"