Team:EPF Lausanne/Project immuno
From 2010.igem.org
| Line 29: | Line 29: | ||
In parallel to the immunotoxin we thought of a different way of blocking the <i>P.falciparum</i> by using a class of proteins, the [https://2010.igem.org/wiki/index.php?title=Team:EPF_Lausanne/Project/Background p-proteins], which are expressed on the membrane of of Plasmodium. These proteins are mainly expressed on the mosquito-stage parasite (ookinete). | In parallel to the immunotoxin we thought of a different way of blocking the <i>P.falciparum</i> by using a class of proteins, the [https://2010.igem.org/wiki/index.php?title=Team:EPF_Lausanne/Project/Background p-proteins], which are expressed on the membrane of of Plasmodium. These proteins are mainly expressed on the mosquito-stage parasite (ookinete). | ||
| - | |||
| - | |||
| - | |||
[[Image:Wiktor1.png|left|250px|caption]] | [[Image:Wiktor1.png|left|250px|caption]] | ||
| - | + | The Plasmodium requires the P-proteins in order to interact with the epithelium and pass through it. | |
| - | The Plasmodium | + | |
| - | + | ||
| Line 43: | Line 38: | ||
| - | + | The idea is to make Asaia express a large amout of these proteins This will cause the interactions of t be also disrupted and the plasmodium will not propagate throught the mosquito's epithelium. | |
Revision as of 18:37, 27 October 2010


Contents |
Proteins
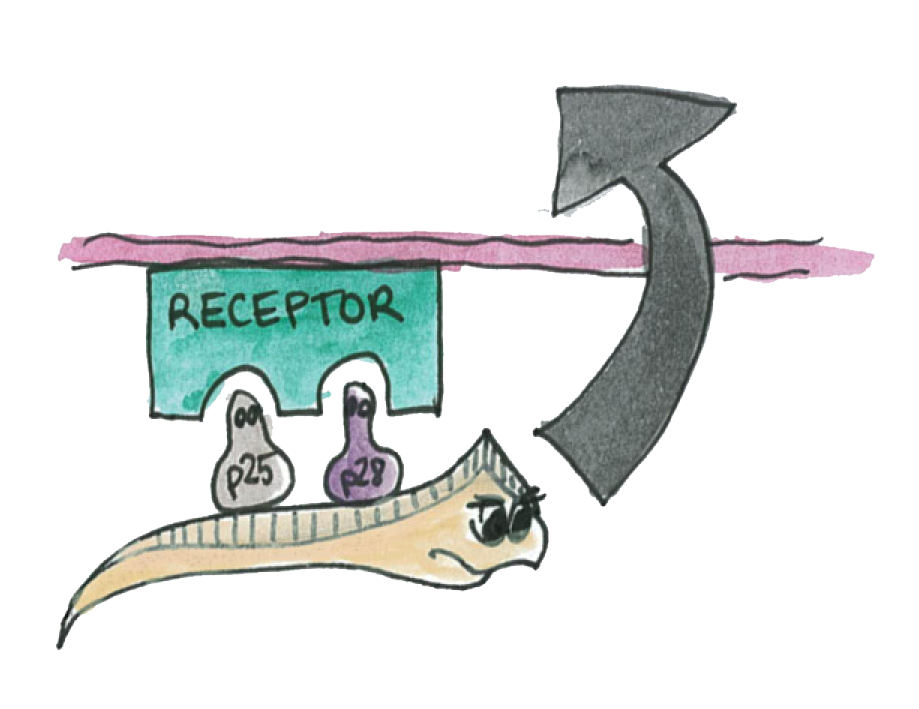
We have chosen two different ways to target the parasite P.falciparum and prevent the malaria transmission through mosquitos. Our engineered bacteria could express either an immunotoxin, or two p-proteins, or even both for maximum efficiency. We tried to express all of these proteins using the C3 plasmid incorporating the strong promoter, a constitutive sequence for greater level of expression.
The Immunotoxin is one of our tools to block transmission of malaria parasite in mosquitos. It is composed of two main parts : The first one is a single-chain antibody fragment (scFv) directed to Pbs2l, which is a surface membrane protein of Plasmodium berghei . The second part is a lytic peptide, Shiva-1, which acts by forming “pores” on the parasite’s membrane. The immunotoxin is supposed to specifically target and lyse the parasite.
In parallel to the immunotoxin we thought of a different way of blocking the P.falciparum by using a class of proteins, the p-proteins, which are expressed on the membrane of of Plasmodium. These proteins are mainly expressed on the mosquito-stage parasite (ookinete).
The Plasmodium requires the P-proteins in order to interact with the epithelium and pass through it.
The idea is to make Asaia express a large amout of these proteins This will cause the interactions of t be also disrupted and the plasmodium will not propagate throught the mosquito's epithelium.
Results
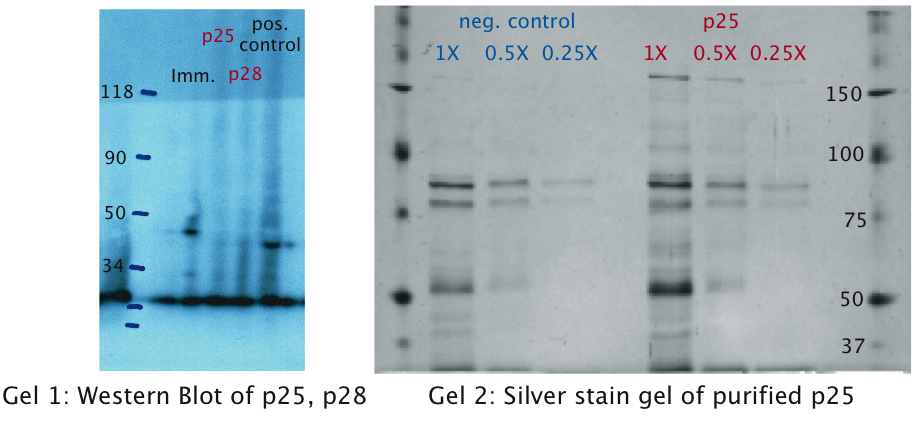
A: The Immunotoxin is expressed and appears in the supernatant
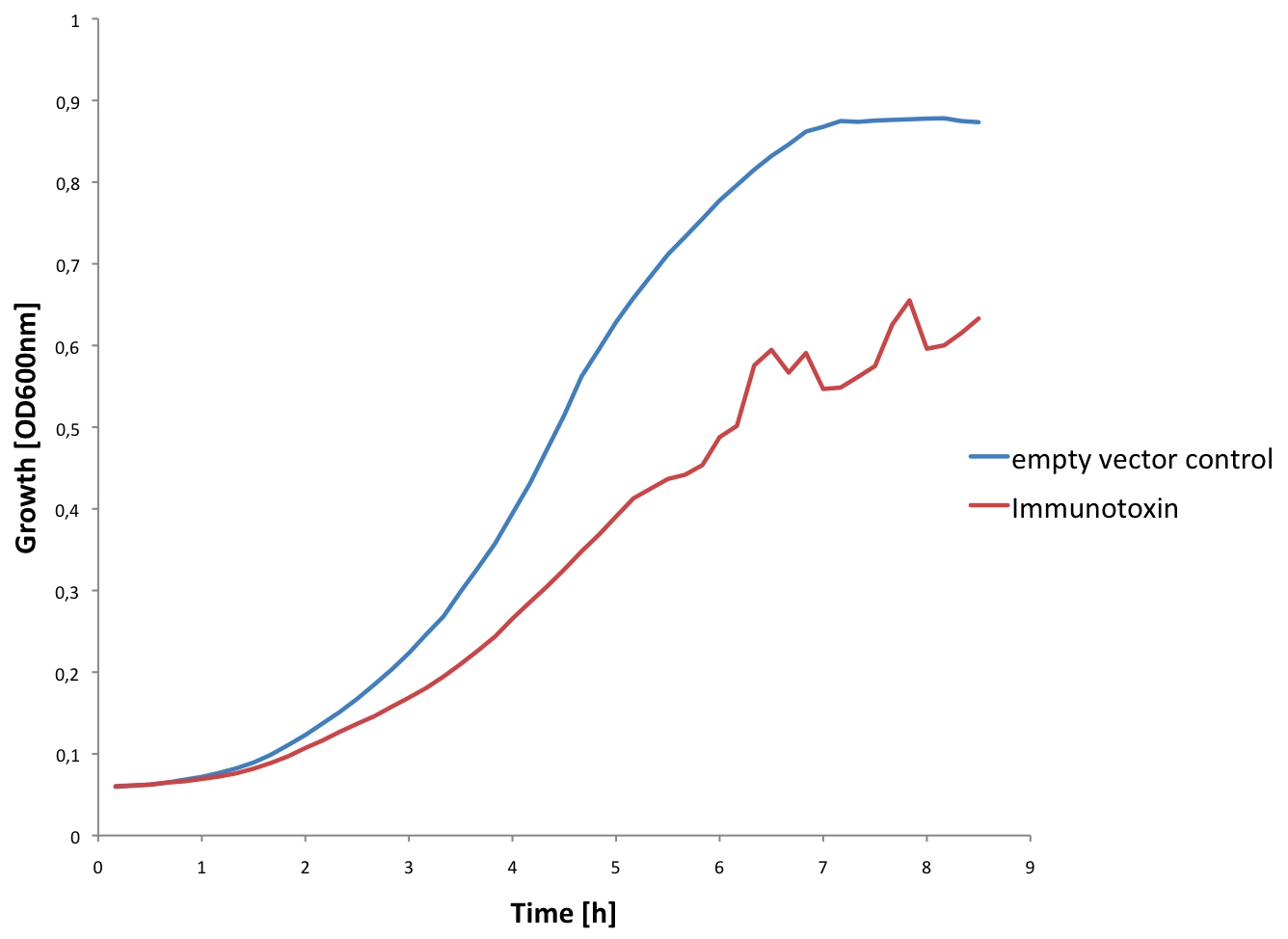
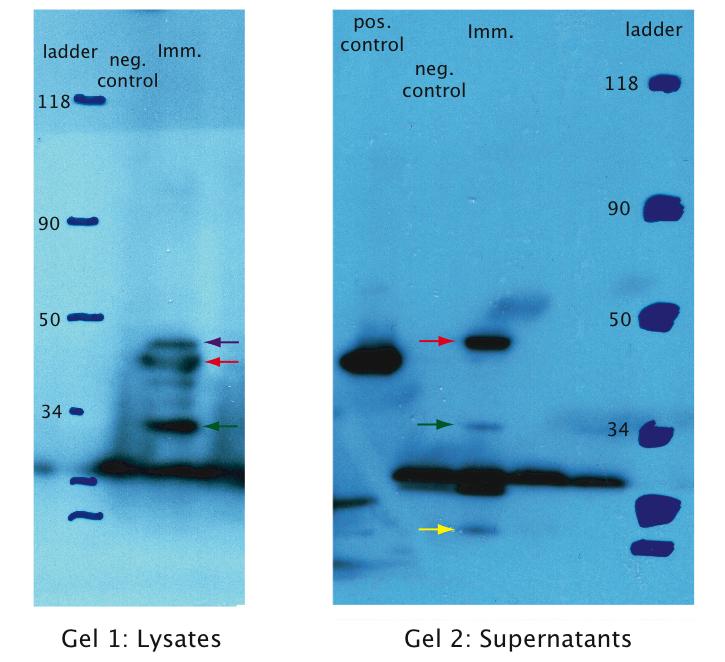
We tested expression of the immunotoxin in E.Coli (see Materials and Methods for details). In a western blot analysis of whole cell lysates we could see bands corresponding to full length immunotoxin and possibly degraded fragments of the protein (see figure). The immunotoxin contains a PelB sequence that targets it for secretion into the periplasm. We concentrated the supernatant of both the immunotoxin and a control culture by running it through a filtering device with a 5 kDa cut-off. Running a western blot with these samples (see figure) we verified that the immunotoxin was found in the supernatant as expected.
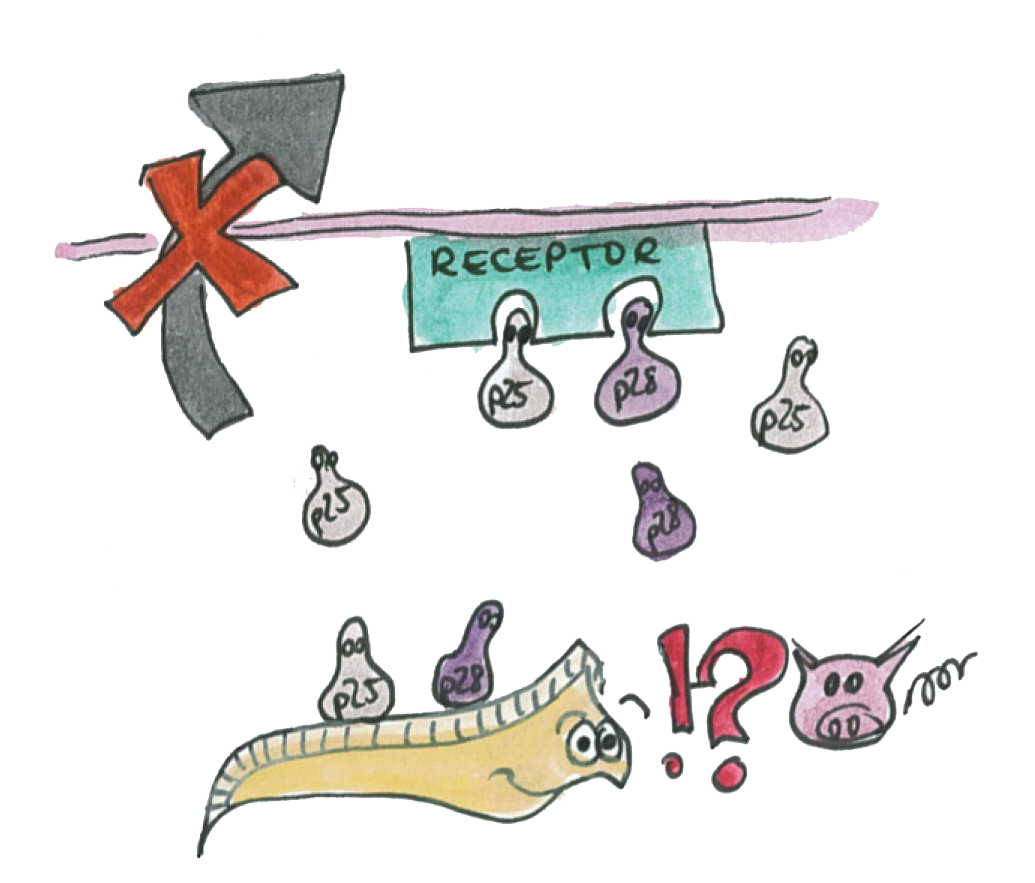
B: The P-proteins are not expressed
The same experiments were conducted for the proteins p25 and p28. No bands were detected on the western blots (see figure) which leads us to the conclusion that these proteins were only very weakly expressed or not at all. This might be explained by the fact that we took the native sequence from Plasmodium falciparum . The genome of Plasmodium falciparum is very A-T-rich ([http://areslab.ucsc.edu/cgi-bin/hgGateway UCSC Malaria Genome Browser]). We think that expression of p25 and p28 may be improved by codon optimizing it for expression in bacteria like E.Coli and Asaia like we did for the immunotoxin. Additional to the Western Blots, to rule out the possibility that concentration was too low, we did a protein purification (following the [http://openwetware.org/wiki/Knight:Purification_of_His-tagged_proteins/Denaturing Knight protocol]) from a large culture volume (see figure) using Ni-NTA columns.

 "
"