Team:Chiba/System 1/Result
From 2010.igem.org
(→Requirement of realizing genetic double click system) |
(→1-2. Pulse generator) |
||
| Line 267: | Line 267: | ||
- 'Invertimer' should create enough time to clear off the repression by CI | - 'Invertimer' should create enough time to clear off the repression by CI | ||
| - | ===<font size=" | + | ===<font size="5"> GFP Pulse Generator Result</font>=== |
----- | ----- | ||
*[http://partsregistry.org/wiki/index.php?title=Part:BBa_K396006 BBa_K396006] | *[http://partsregistry.org/wiki/index.php?title=Part:BBa_K396006 BBa_K396006] | ||
Revision as of 03:11, 28 October 2010

Version 1 :
Successful operation of this system requires
1. Generator of T7 RNA Polymerase pulse
In response to both the 1st and the 2nd input, T7 RNAP has to be effective for a moment then
get silent. To fulfill this task, - Lux/CI434 hybrid promoter: -must be activated by LuxR in the presence of AHLs
-must be strictly repressed by CI434
-must NOT be repressed by CI (another repressor components in the circuit)
- CI434 -should be expressed upon AHL input
-must become effective after a certain time (delayed repression)
-should tightly repress
- T7RNAP - must be cleared off as soon as possible when its expression is stopped
2. Output module (AND gates of T7RNAP & (NOT CI))
- T7/CI promoter should be activated by T7 RNAP - T7/CI promoter should be tightly repressed by CI - CI should be degraded off after expression shutted off(upon AHL input)
3. Tuning the timing.
- During the T7 RNAP pulse, CI should keep its repression power.
( T7RNAP pulse should be gone before repression by CI start decreasing)
- 'Invertimer' should create enough time to clear off the repression by CI
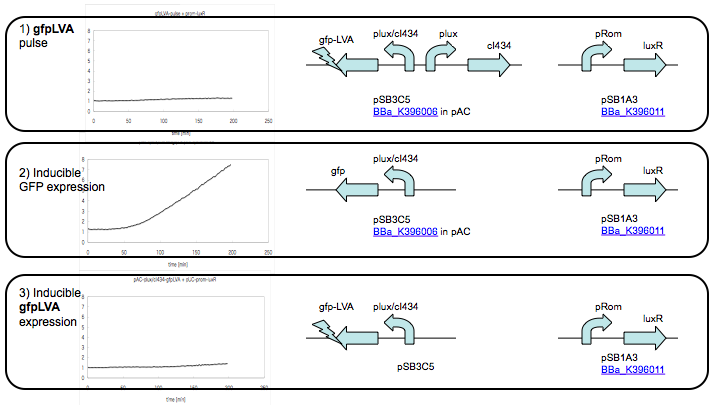
GFP Pulse Generator Result
Protocol
E. coli strain DH10B was used for the pulse-generator experiments. Co-transformed cell were pre-cultured in test tubes for overnight at 37C, 200rpm. Dilute the culture 1:100 into 2ml of fresh medium and grow for an additional 5 hours in Supplemented M9 medium . For the liquid experiments, expression was induced at the log phase (OD600 of 0.4) by the addition of AHL (3OC6HSL)at the appropriate concentration. One-milliliter samples were taken every 5 min.
Result
There are no obvious pulse observed.We use destabilized GFP at the downstream of pLux/cI434 hybrid promoter as a positive control, but it also didn't generate any fluorescent.Maybe because we use weak RBS and GFP with LVA, the GFP protein degradation is too soon to be observed. Our project focus on T7 RNA Polymerase pulse and the output GFP will be amplified. So we hope there will be GFP pulse generated when co-transform plasmid 1 and 2.
 "
"