Team:Calgary/11 August 2010
From 2010.igem.org
| Line 1: | Line 1: | ||
{{CalgaryNotebookTemplate| | {{CalgaryNotebookTemplate| | ||
'''Wednesday August 11, 2010''' | '''Wednesday August 11, 2010''' | ||
| + | |||
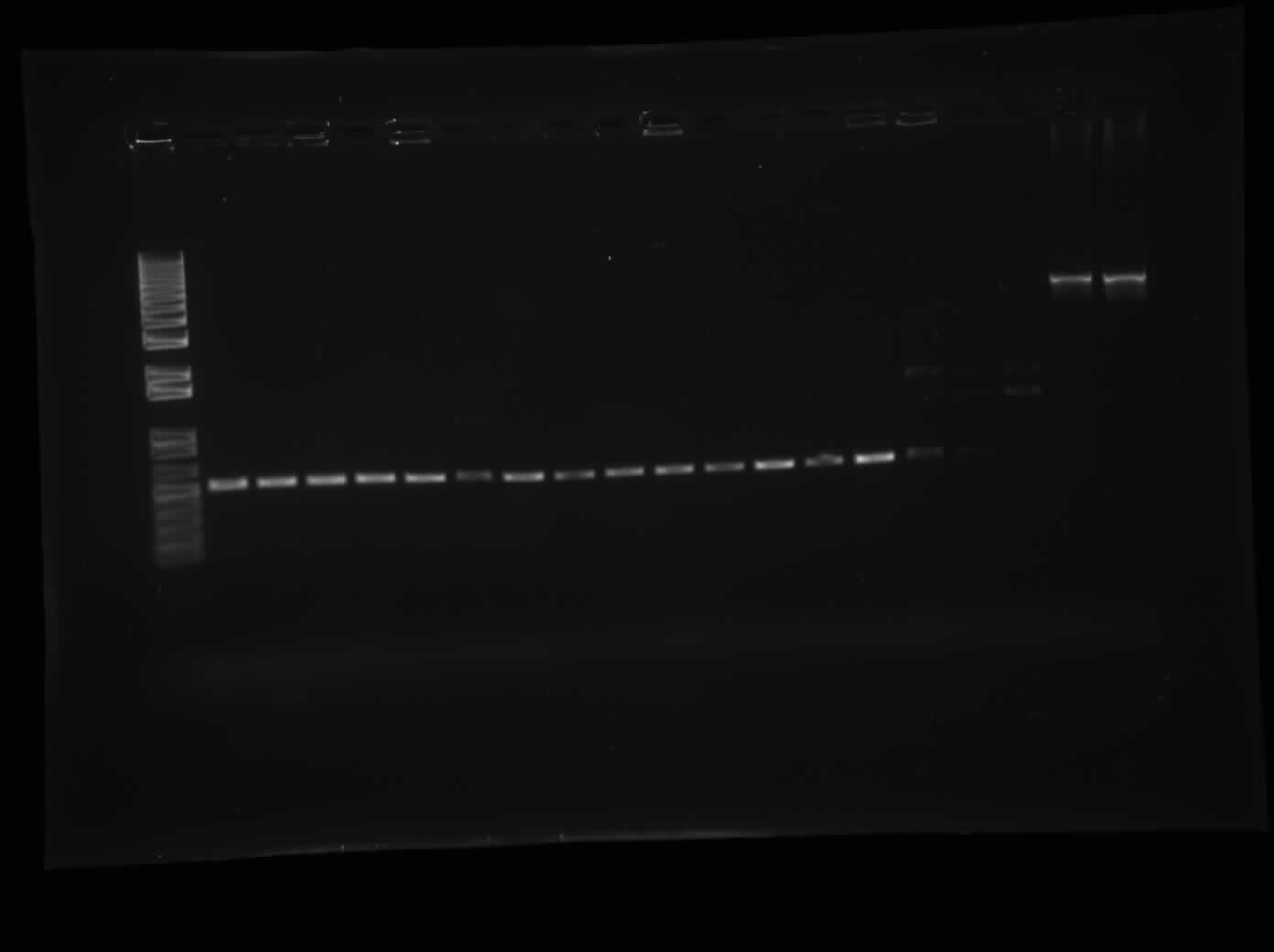
| + | [[Image:08.11.2010-CpxP-MalE.jpg|thumb|400px|Chris's gel of the CpxP promoter with the far right two lanes being Emily's MalE.]] | ||
<u>Jeremy</u> | <u>Jeremy</u> | ||
Revision as of 14:47, 12 August 2010

Wednesday August 11, 2010
Jeremy
Today I did a miniprep of the pRFP colonies because I ran out. Then I biobricked the pRFP using a gradient PCR with an annealing temperature of 55 degrees to 73 with 8 tubes in a 12 well gradient placed evenly. The PCRs that are run include 1.0 mM of MgCl2, 1.5 mM of MgCl2 (regular), 2.0 mM of MgCl2 using the new biobrick primers with NotI and XbaI forward sites and SpeI and NotI reverse sites. The tube of pRFP used was from colony 3 (C3). I also ran a 1.3 mM MgCl2 with the old primers with EcoRI, NotI, XbaI and the SpeI on the reverse. I also did a colony PCR of the pRFP plasmid switch (old PCR purified product and old primers). I ran the gel and the results are..... I also did my article for the blog.
Raida
Today, I ran a 1% agarose gel electrophoresis of my PCR from yesterday. The PCR was done with Ssdeleted forward primer and reverse primer on malE31 template and also malE31 without the signal sequence and MalE as a positive control. The results were positive for malE31delSS and also MalE. So Emily PCR purified the PCR product. The next step will be doing restriction digests and also cloning into a TOPO vector.
Chris
Today, I did 21 plasmid preparations of a variety of different parts including p31δSS, CpxP, MalE, MalE31 and I0500-I13504. The concentrations are available upon request. As well, I ran a 1.0% agarose gel electrophoresis of the colony PCR of 16 different CpxP colonies. The picture of the gel can be seen on the right. The bands are shown at approximately 520 bp, which, with the Biobrick primers, is approximately where they should be. Finally, I made overnight cultures of I13507, I13504 and the ibpABfsxAT7 Promoter in biobrick form.
No notebook page exists for this date. Sorry! "
"