Team:Bielefeld-Germany/Results/Characterization/K389011
From 2010.igem.org
Characterization of <partinfo>K389011</partinfo>
The induction of virA with acetosyringone was tested with different response devices. One of the read out systems was the <partinfo>BB_K389011</partinfo> with a kanamycin resistance cassette under control of the virG promoter. To measure the expression of the resistance gene, hundreds of colonies including <partinfo>BB_K389011</partinfo> and <partinfo>BB_K389010</partinfo> (virA) were transferred to agar plates with rising concentrations of kanamycin. The colonies which were able to grow on each plate were divided by the total number of colonies on a plate without kanamycin to calculate the survival rate φ. The ability of the bacterial population to withstand the antibiotic was determined without any inducing substance and in the presence of acetosyringone. The results of this experiment are summarized in the figure below. The two curves (induced and non-induced population) indicate a common tendency. Under rising concentrations of kanamycin (x-axis) the survival rate of each population (y-axis) first decreases slowly but drops dramatically at a certain concentration leading to the death of all bacteria. This minimal inhibitory concentration (MIC), which completely inhibits visible growth of a population, is higher in the curve of induced bacteria.
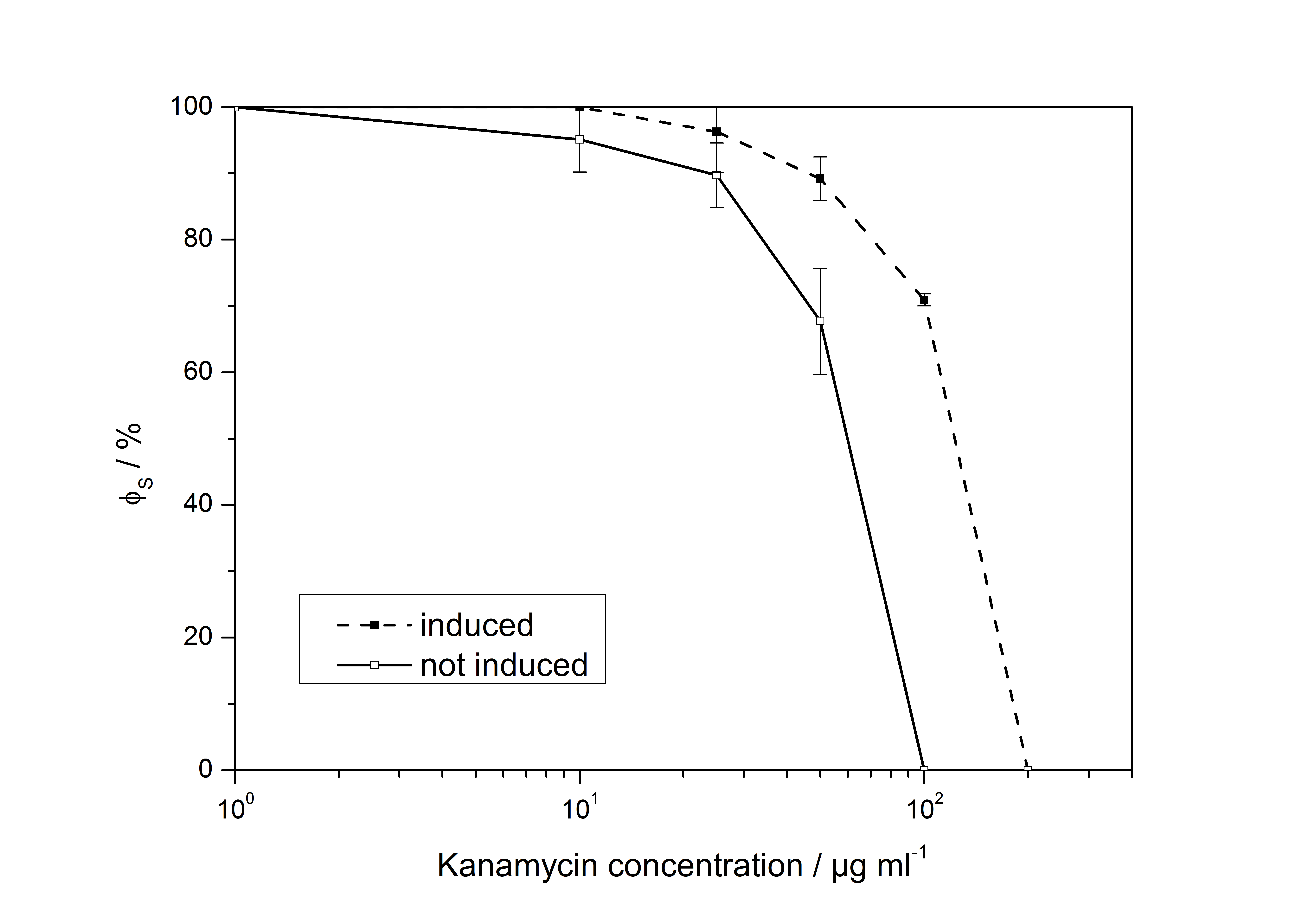
The interpretation of this results leads to two different conclusions.
First, approximately 70 % of the uninduced bacteria can withstand common working concentrations of kanamycin (25 – 50 µg mL-1). This result shows that the whole receptor system seems to be not very tight regulated, causing a relatively high basal transcription. The observed leakiness might either be caused by the presence of transcription factors in E. coli other than VirG that can initiate the transcription at the virB promoter or by a significant activity of the VirA kinase domain without acetosyrigone.
The second result of the determination of MIC of kanamycin is that the induction of VirA can be detected by the growth of colonies on agar plates with 100 µg mL-1. As indicated in the figure none of the uninduced bacteria could grow at this concentration, while more than 70 % of the induced population showed a sufficient expression of the resistance cassette.
According to these results, we chose to use a kanamycin selection system during our directed mutagenesis. The idea was to select bacteria with mutated virA variants that can be activated by substances other than acetosyringone (e.g. capsaicin) by their growth on agar plates with 100 µg mL-1 kanamycin.
 "
"


