Team:Berkeley/Project/Self Lysis
From 2010.igem.org
| Line 38: | Line 38: | ||
''Media Characterization''<br> | ''Media Characterization''<br> | ||
| - | But switching out a promoter didn't end our battle to apply this construct to a new system: we had to find a media that would be an appropriate environment for the E. coli and Choanoflagellates. Berkeley 2008 characterized the part in LB, but Choanoflagellates are sluggish and generally unhealthy in LB. Thus, we had to find another media in which to perform our delivery scheme. | + | But switching out a promoter didn't end our battle to apply this construct to a new system: we had to find a media that would be an appropriate environment for both the E. coli and Choanoflagellates. Berkeley 2008 characterized the part in LB, but Choanoflagellates are sluggish and generally unhealthy in LB. Thus, we had to find another media in which to perform our delivery scheme. |
| Line 44: | Line 44: | ||
<font size="5">'''Characterization in new medias'''</font> | <font size="5">'''Characterization in new medias'''</font> | ||
| - | The 2008 team already characterized the device in LB, as shown here (insert link). However, | + | The 2008 team already characterized the device in LB, as shown here (insert link). However, choanoflagellates cannot survive in TB or LB media. Since we are applying the device to a new setting, we needed to characterize it in new conditions. Our challenge was to find a media that would both allow lysis to occur and keep the Choanoflagellates happy and healthy. |
<font size=3>'''Medias Tested'''</font> | <font size=3>'''Medias Tested'''</font> | ||
| Line 90: | Line 90: | ||
[[Image:media assay chart.png | 200px]] | [[Image:media assay chart.png | 200px]] | ||
| - | The best media proved to be CGM, Cereal Grass Media, because it both kept the Choanoflagellates healthy and allowed lysis to occur. Thus, although we continued to culture our Choanoflagellates in ASW and grow up our E. coli in TB, we performed all of our payload delivery assays in CGM | + | The best media proved to be CGM, Cereal Grass Media, because it both kept the Choanoflagellates healthy and allowed lysis to occur. Thus, although we continued to culture our Choanoflagellates in ASW and grow up our E. coli in TB, we performed all of our payload delivery assays in CGM. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 21:46, 26 October 2010
- Home
- Project
- Parts
- Self-Lysis
- Vesicle-Buster
- Payload
- Parts Submitted
- Results
- Judging
- Clotho
- Human Practices
- Team Resources
Overview
The Self Lysis Device acts to degrade the bacterial membrane (inner membrane, peptidoglycan layer and outer membrane). In the overall delivery scheme, if the timing works out so that self-lysis occurs after the bacteria has been consumed by the Choanoflagellate, the payload will be released into the vesicle.
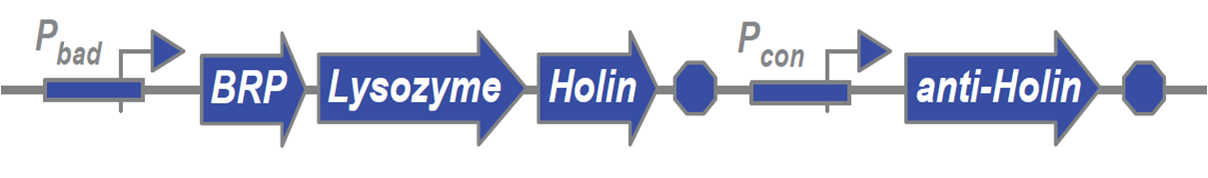
The Construct
- Pbad promoter: allows for fast, controlled induction, which allows us to better control the timing of lysis. Also, since this promoter is only induced by an exogenous molecule, it helps address biosafety concerns.
- Bacteriocin Releasing Protein (BRP): degrades the outer membrane
- Lysozyme: degrades the peptidoglycan layer
- Holin: creates pores in the inner membrane
- Anti-holin: threshold-gating system
(for more complete descriptions, see 2008 Berkeley iGEM team's wiki)
Our Story: From 2008 to 2010
Our project involved using a modified version of the 2008 Berkeley iGEM construct in an entirely new, innovative setting. The 2008 team hoped that Self-lysis would be a means of product purification and characterized the part in a standard E. coli media, Luria Broth (LB), as outlined here. In the Anderson lab, the construct was modified to include the BRP protein and has been extensively tested as a means of delivery in Mammalian cells. We, the 2010 Berkeley iGEM team, are using this device in an entirely new setting: the single celled, lower metazoans called Choanoflagellates.
The Magnesium Promoter
Our team started out by using a version of the Self-lysis device that was under a Magnesium promoter, which turns on in low levels of Mg. This construct has been used extensively in the Anderson lab for projects in Mammalian delivery, since it is a means for the E. coli to detect when it has been encased in a phagocytic vesicle and thus trigger self-lysis in the appropriate setting. However, since this promoter takes about 4 hours to turn on and we soon realized that Choanoflagellates eat and digest bacteria along with its payload in about an hour, this promoter is much too slow for our delivery scheme. Thus, we decided to switch out the Magnesium promoter for a Pbad promoter, which allows Self-lysis to occur within an hour after induction.
Media Characterization
But switching out a promoter didn't end our battle to apply this construct to a new system: we had to find a media that would be an appropriate environment for both the E. coli and Choanoflagellates. Berkeley 2008 characterized the part in LB, but Choanoflagellates are sluggish and generally unhealthy in LB. Thus, we had to find another media in which to perform our delivery scheme.
Characterization in new medias
The 2008 team already characterized the device in LB, as shown here (insert link). However, choanoflagellates cannot survive in TB or LB media. Since we are applying the device to a new setting, we needed to characterize it in new conditions. Our challenge was to find a media that would both allow lysis to occur and keep the Choanoflagellates happy and healthy.
Medias Tested
- Luria Broth (LB): Positive Control, used to confirm the results of the iGEM 2008 team and to make sure the assembly with the Vesicle Buster Device didn't disrupt lysis, since it tends to be a fairly tempermental part.
- Terrific Broth (TB): Used to grow up our payload delivery device and payload containing bacteria to a high concentration before feeding them to the Choanos. E. coli flourish in TB.
- Cereal Grass Media (CGM): Developed by the King lab to culture Choanos.
- Artificial Sea Water (ASW): Sterilized salt water that we use to culture our line of Choanos.
- Different ASW:LB ratios
- Spring water and wheat seed medium (used to culture many fresh water eukaryotes)
- Different Spring water:LB ratios
Additional information about CGM and ASW can be found here.
Testing lysis
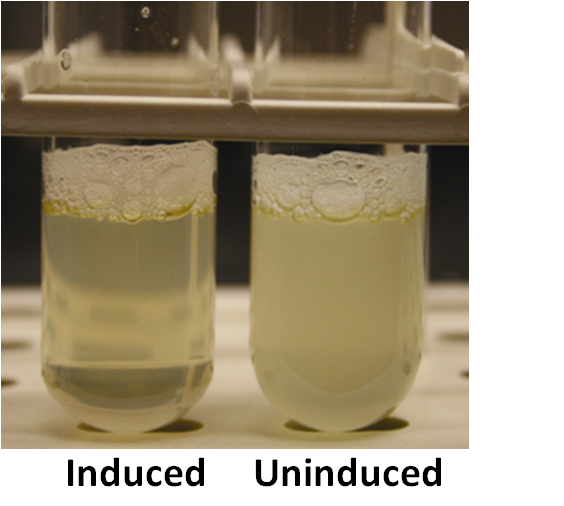
Self-lysis can be assayed by measuring changes in optical density, or changes in the opaqueness of a solution. As lysis occurs, optical density decreases, meaning the solution of bacteria becomes more translucent.
On a macro-scale, here's what you'd expect to see from a working self-lysis device:
We used a TECAN machine to measure the change in optical density over time. The following data points are averages from several runs on colonies from the same transformation. The data has been normalized (insert details) and show error bars (insert details!).
TECAN DATA
Thus, TB is the best media for lysis, followed by LB, CGM3, high ratios of LB:ASW, low ratios of LB: ASW, and ASW. While no TECAN data is available, macroscopic tests indicate that lysis occurs in spring water:LB ratios as high as 80:20.
Observing Choanoflagellate health
We observed the Choanoflagellates after incubating them in TB and LB, and they appeared to be very sluggish, which is a sign that they are unhealthy.
Choanos at 37deg?? Conor's data?
CONOR's DATA
Conclusion
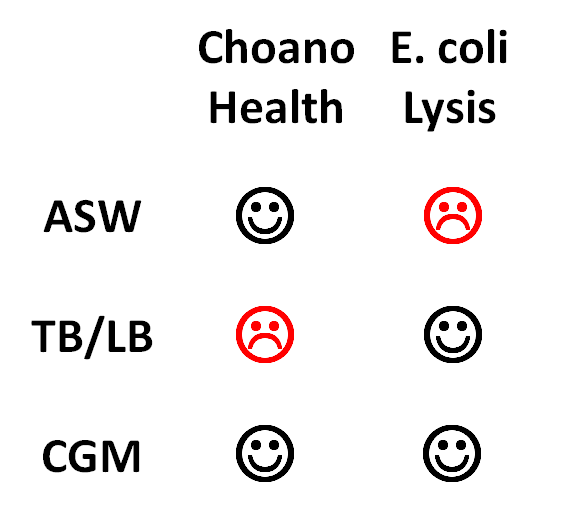
This table sums up the results of our lysis and Choano health assays:
The best media proved to be CGM, Cereal Grass Media, because it both kept the Choanoflagellates healthy and allowed lysis to occur. Thus, although we continued to culture our Choanoflagellates in ASW and grow up our E. coli in TB, we performed all of our payload delivery assays in CGM.
 "
"