Team:Berkeley/Human Practices
From 2010.igem.org
| Line 1: | Line 1: | ||
| + | [[Image:human practices header]] | ||
| + | |||
<!-- Templates. Should be included on every "main" page --> | <!-- Templates. Should be included on every "main" page --> | ||
{{Berkeley_Main_Script}} | {{Berkeley_Main_Script}} | ||
Revision as of 01:26, 27 October 2010
Human Practices: Rethinking Biosafety
Current Biosafety Standards
Biosafety is undoubtedly one of the highest priorities of any synthetic biology institution. When manipulating systems as complex as the cell, there is always high degree of uncertainty. Unexpected results in living constructs have the potential to be disastrous, especially when dealing with pathogenic organisms or virulence factors. The NIH and CDC have developed a standard of biosafety levels (BSL) to rate the risk factor associated with different biological agents and genetic parts. They are summarized here:
- BSL 1- Well characterized parts and organisms that are not consistently pathogenic to healthy adults.
- BSL 2- Associated with mild human diseases, but do not readily spread through aerosols
- BSL 3- Associated with deadly or severe human diseases that we have vaccines or treatments for.
- BSL 4- Associated with deadly, aerosol-spread diseases that have no known vaccine or treatment
The current system of rating synthetic biological devices simply chooses the highest rated part or gene within the device and rates the entire device based on that number. For example, we could not submit our Vesicle Lysis parts this year because they contained genes from the pathogen "Clostridium perfringens", making the entire device BSL2.
Shortcomings of the System
As synthetic biology becomes more sophisticated, and genes from various organisms are mixed to create novel tools,
the threat of creating dangerous constructs from innocent parts becomes real. We encountered this very danger in our product. We created an engineered organism that used a Self Lysis device (made up of BSL1 parts) to release its contents inside vesicles and a Vesicle Buster device (which contains BSL2 parts)to break out of any cholesterol-based vesicle membrane. This device has been shown to function in mammalian cells. Lastly, we constructed a number of transposase devices (all BSL1) to potentially integrate transposons into our target's DNA. If a bacteria containing these three unrelated devices entered the body and was exposed to a macrophage, it could quite possibly break into a human cell, deliver its payload and carcinogenically alter the human genome. Fortunately, this risk was realized long before any parts were built this summer, and the necessary precautions were taken. This risk was one of the driving forces for putting our lysis device under inducible control.
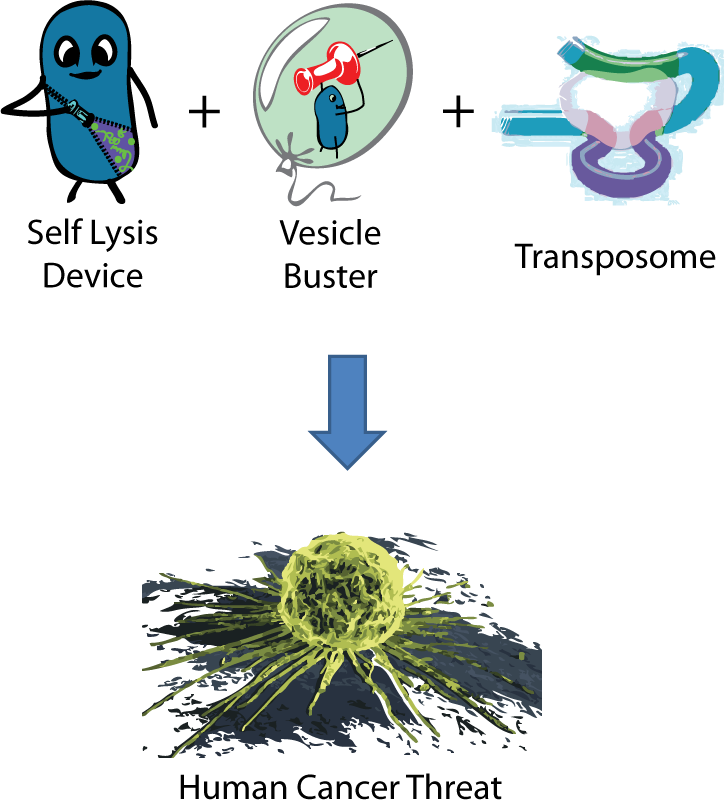
The discovery of this potential risk illuminated that the current biosafety rating system cannot identify potential threats from the combination of seemingly harmless genetic parts. This is disconcerting, considering how the culture of synthetic biology is moving towards fast-paced construction and exchange of genetic parts, with less and less consideration for the original source of such parts. This could be dangerous to the scientists who are ignorant of the virulence they are synthesizing. The failure of government standards to recognize engineered threats also means that researchers could intentionally synthesize virulence while avoiding regulations and restrictions that should be required in such risky situations.
 "
"