| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 70s
| 30 cycles
|
| 72
| 300s
|
- PCR of split GFP (use pfu/4 tubes)
| Total: | 50μl | X2μl | | | FP | RP | Template
|
| Template | 1μl | 2μl | | negative | VF2 | VR |
|
| FP | 1μl | | | positive | VF2 | VR | complete GFP
|
| RP | 1μl | | | GFP_A | GFP_split_A_FP | GFP_split_A_RP | complete GFP
|
| dNTP | 4μl | 8μl | | GFP_B | GFP_split_B_FP | GFP_split_B_RP | complete GFP
|
| 10XBuff. | 5μl | 10μl | |
|
| pfu | 1μl | 2μl |
|
| ddH2O | 37μl | 74μl |
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 150s
| 35 cycles
|
| 72
| 300s
|
2010.08.10
- PCR of GFP (A+B)
- To make sure the original GFP+eIF4A can emit fluorescence.
- Gel extration >> purification of split GFP(A.B)
- PCR of GFP(A+B) (use pfu/3 tubes)
| Total: | 50μl | X1.5μl | | FP | RP
|
| Template | 1μl | | negative | |
|
| FP | 1μl | | positive | VF2 | VR
|
| RP | 1μl | | GFP(A+B) | GFP_split_A_FP | GFP_split_B_RP
|
| dNTP | 4μl | 6μl |
|
| 10XBuff. | 5μl | 7.5μl |
|
| pfu | 1μl | 1.5μl |
|
| ddH2O | 37μl | 56.25μl |
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 150s
| 35 cycles
|
| 72
| 300s
|
2010.08.12
- PCR of aptamer
- PCR >> Gel extraction >> purification
- PCR (use pfu/3 tubes)
| Total: | 50μl | X2μl | | FP | RP | length
|
| FP | 1μl | | negative | | |
|
| RP | 1μl | | positive(pTet-1) | VF2 | VR | 292
|
| dNTP | 4μl | 8μl | aptamer | aptamer_FP | aptamer_RP | 96
|
| 10XBuff. | 5μl | 10μl
|
| pfu | 1μl | 2μl
|
| ddH2O | 38μl | 76μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 40s
| 35 cycles
|
| 72
| 300s
|
2010.08.13
- digestion,PCR purification,ligation and transformation(aptamer on pSB1A2)
- digestion of aptamer (XP) >> PCR purification >> ligation(aptamer+pSB1A2 from YFP) >> transfomation
- digestion protocol
| Total | 30μl
|
| DNA | 26μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| Total | 10μl | length
|
| ligase | 0.5μl |
|
| ligation buffer | 1μl |
|
| DNA1(aptamer(XP)) | 8μl | 88
|
| DNA2(pSB1A2) | 0.5μl | 2100
|
2010.08.16
- 3 in 1 of aptamer on pSB1A2
- PCR protocol for test(5tubes)
| Total: | 50μl | X3.6μl
|
| Template | 1μl |
|
| FP | 1μl | 3.6μl
|
| RP | 1μl | 3.6μl
|
| dNTP | 2μl | 7.2μl
|
| 10XBuff. | 5μl | 18μl
|
| taq | 0.25μl | 0.9μl
|
| ddH2O | 39.75μl | 143.1μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
2010.08.17
- plasmid extraction of aptamer on plasmid and digestion aptamer SP
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (S) | 1μl
|
| Enzyme 2 (P) | 1μl
|
2010.08.18
- gel extraction >> purification >> ligation >> transformation of aptamer(SP)
- Transformation failed, so we digest again on 8/20. (no colonies)
- ligation protocol
| Total | 10μl | length
|
| ligase | 0.5μl |
|
| ligation buffer | 1μl |
|
| DNA1(aptamer(SP)) | 3μl | 332
|
| DNA2(terminator) | 5.5μl | 129
|
2010.08.20
- digestion of aptamer(SP) and terminator(XP)
- Do gel extraction, and when running electrophoresis we found that digestion failed so we restart to digest again.
2010.08.21
- gel extraction and purification of aptamer(SP)
- We checked the result of digestion and aptamer succeed but terminator failed.
- continued to purify aptamer(SP), and ligation aptamer(SP) and terminator(XP,from SSrA)
- transformation
2010.08.22
- 3 in 1 of aptamer+terminator
- colony PCR and run electrophoresis
- negative was contaminated
2010.08.23
- colony PCR of aptamer+terminator and plasmid extraction
- check result of 8/22 but contaminated
- restart to digest terminator(XP) and aptamer+terminator(XP)
2010.08.24
- aptamer purification and eIF4A PCR
- We run electrophoresis to check and terminator(XP) succeed, aptamer+terminator(XP) failed.
- eIF4A PCR(use tag)
2010.08.25
- 3 in 1 of aptamer and eIF4A electrophoresis(and design primer)
2010.08.26
- plasmid extraction,digestion and purification of apt.+ter.
- result of extraction → 3.4.5 be poluted,so use 1.2. for digest
- digestion protocol(apt.+ter.)(XP)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- electrophoresis of tag Gradient(eIF4A) and apt.+ter.
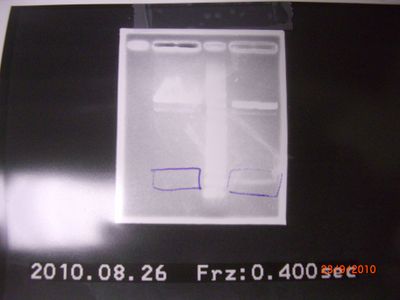 left → tube 1. right → tube 2. 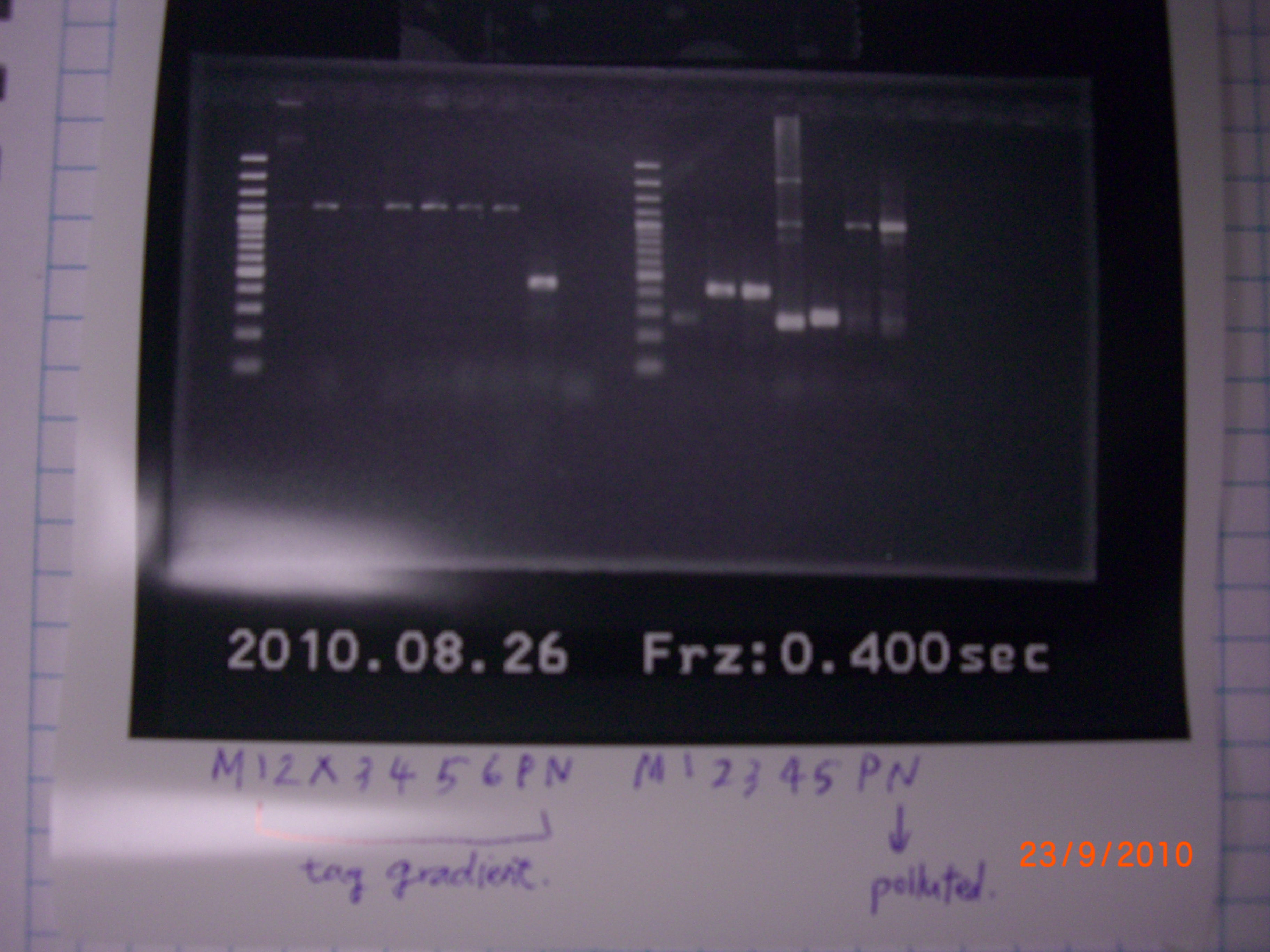 the result of check → It is possible for VR.VF2 to be polluted. - also purification of RBS
- PCR of complete eIF4A → purification→ digestion(XP)→ ligation with pSB1A2(XP) → transformation
- real PCR solution of complete eIF4A(X2)(take out at 18:50)
| Total: | 50μl | X2μl | | | FP | RP | Template
|
| Template | 1μl | | | negative | VF2 | VR |
|
| FP | 1μl | 2μl | | positive | VF2 | VR | complete GFP
|
| RP | 1μl | 2μl |
|
| dNTP | 4μl | 8μl |
|
| 10XBuff. | 5μl | 10μl |
|
| pfu | 1μl | 2μl |
|
| ddH2O | 37μl | 74μl |
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 15s
|
| 53.6
| 20s
|
| 72
| 90s
| 35 cycles
|
| 72
| 60s
|
- ligation protocol(10mins)
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(eIF4A[XP]) | 3μl
|
| DNA2(pSB1A2[XP]) | 1μl
|
| ddH2O | 4.5μl
|
- transform(competent cell 30ul)
- ligation of [apt.+ter.]+RBS
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(apt.+ter[XP]) | 7.7μl
|
| DNA2(RBS[SP]) | 0.8μl
|
2010.08.27
- colony PCR of apt+ter+RBS
- colony PCR of eIF4A+pSB1A2
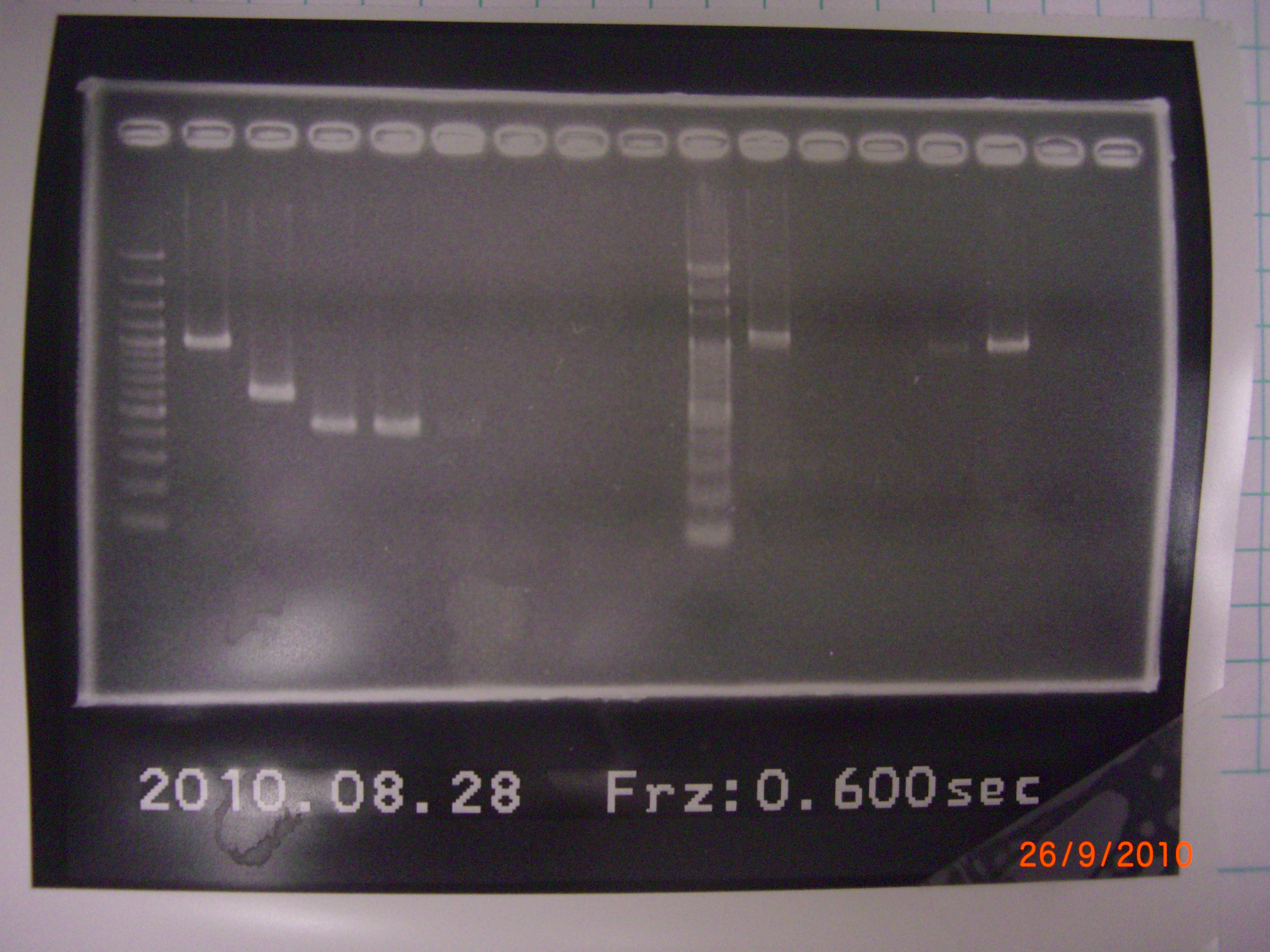
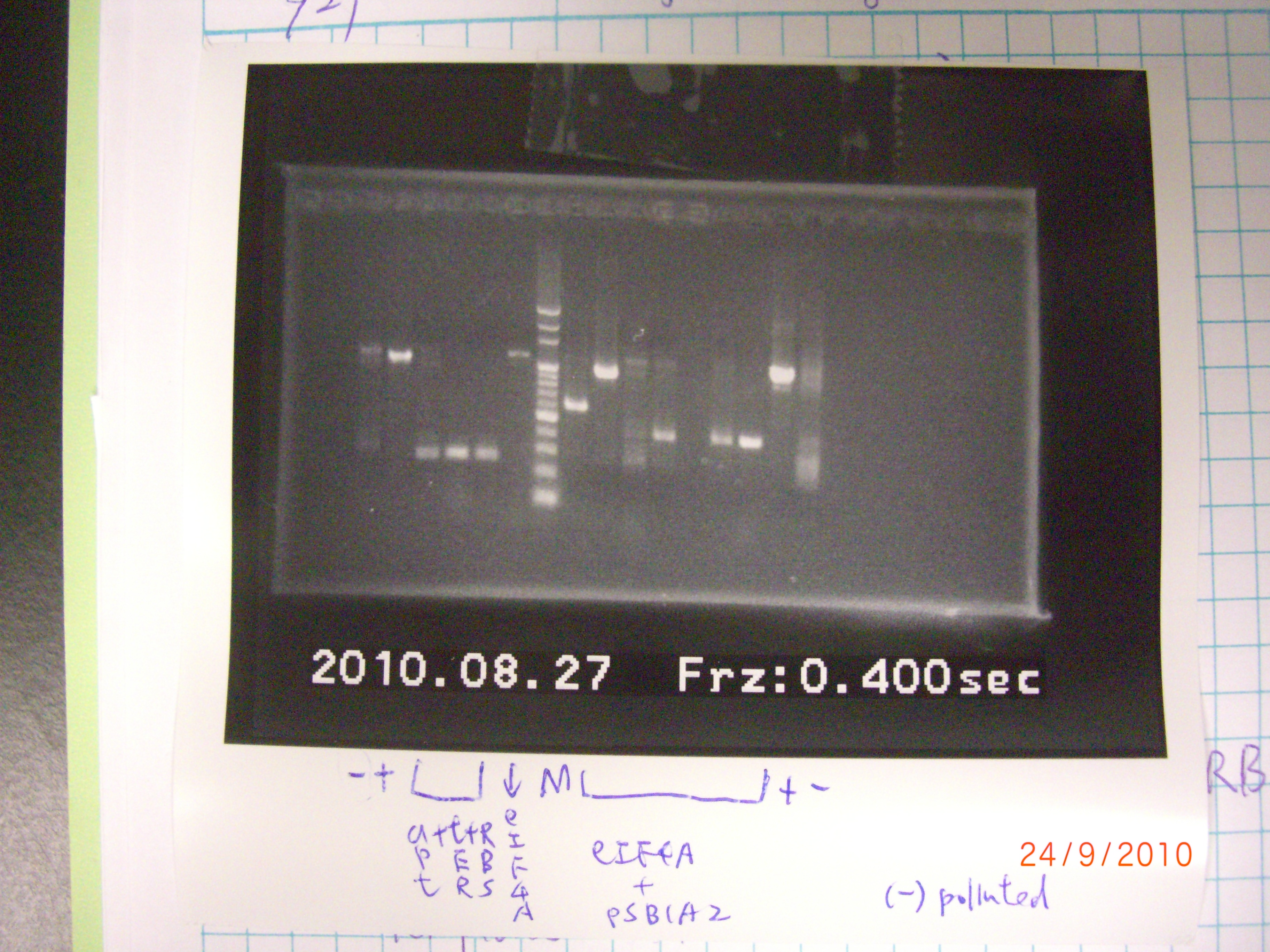 result of both of the PCR failed, it is possible for the negative control to be contaminated. We change the system of colony PCR, and PCR the eIF4A+pSB1A2 again. 2010.08.28
2010.08.29
- plasmid extraction of eIF4A on pSB1A2 and apt.+ter.+RBS
- digestion of eIF4A on pSB1A2(XP)[check], apt.+ter.+RBS(XP), pLac(SP) and eIF4A(XP)
- digestion protocol of eIF4A on pSB1A2(XP) and apt.+ter.+RBS(XP)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
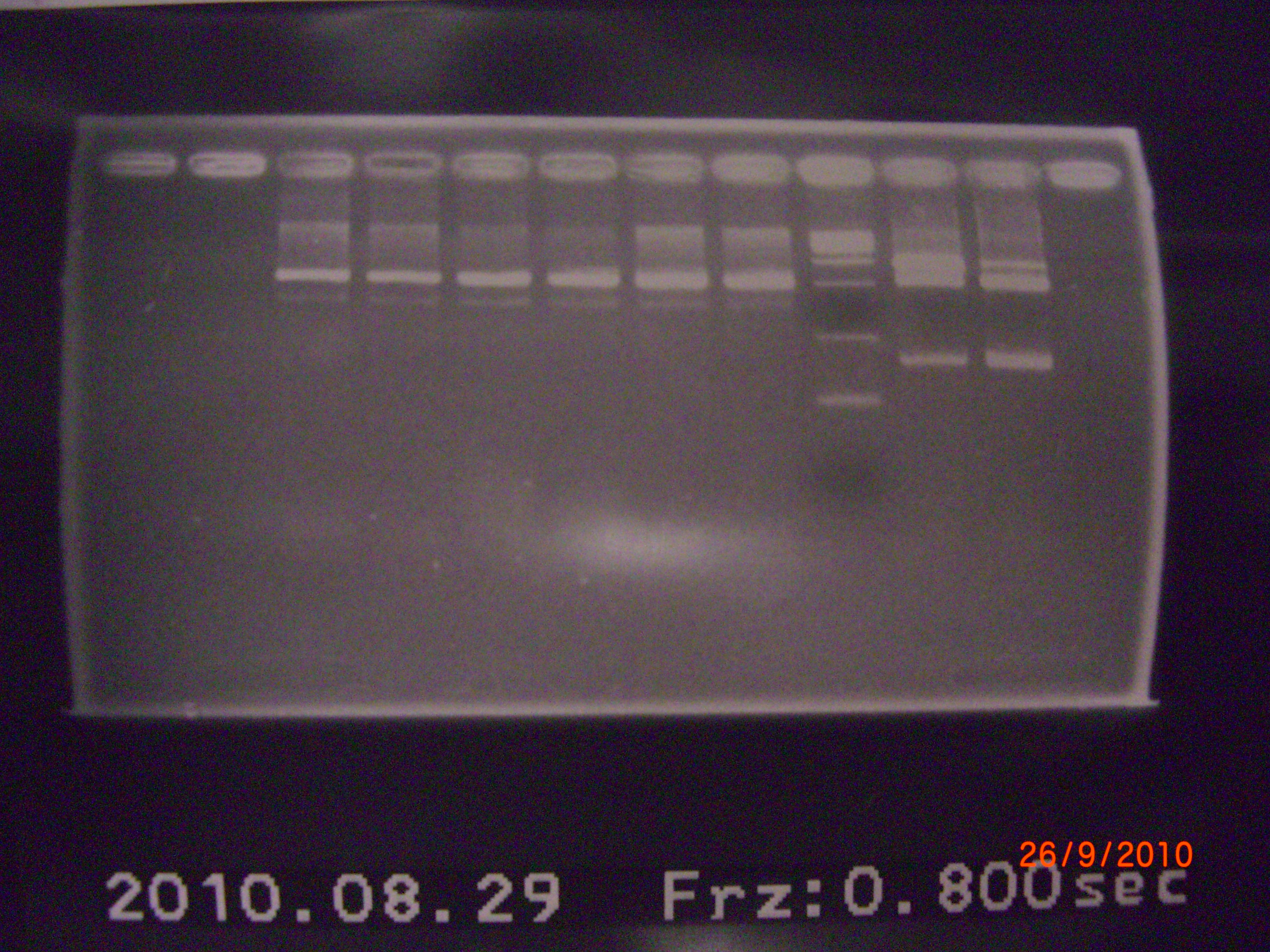 bend1~6 → apt.+ter.+RBS(XP) // maker // eIF4A on pSB1A2(XP) // result of check is that (apt.+ter.)+(RBS) did not ligase, so we decided to ligase them again. - digestion protocol of pLac(SP) and eIF4A(XP)
| Total | 20μl
|
| DNA | 10μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (S) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 6μl
|
- purification of eIF4A(XP) and pLac(SP)
- ligation of (apt.+ter/XP)+(RBS/SP)
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(apt.+ter[XP]) | 7.7μl
|
| DNA2(RBS[SP]) | 0.8μl
|
- transformation of the above one
2010.08.30
- We discover that eIF4A sequence has 2 PstI enzyme cutting sites, so we design primer and make a eIF4A sequence by ourselves.
- There are no colonies on the transformation plate yesterday(apt.+ter(XP))+(RBS(SP)), so we digest the apt.+ter/XP and RBS/SP for the beginning.
- digestion protocol of apt.+ter(XP)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- digestion protocol of RBS(SP)
| Total | 20μl
|
| DNA | 10μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddddH2O | 6μl
|
- run gel and gel extraction
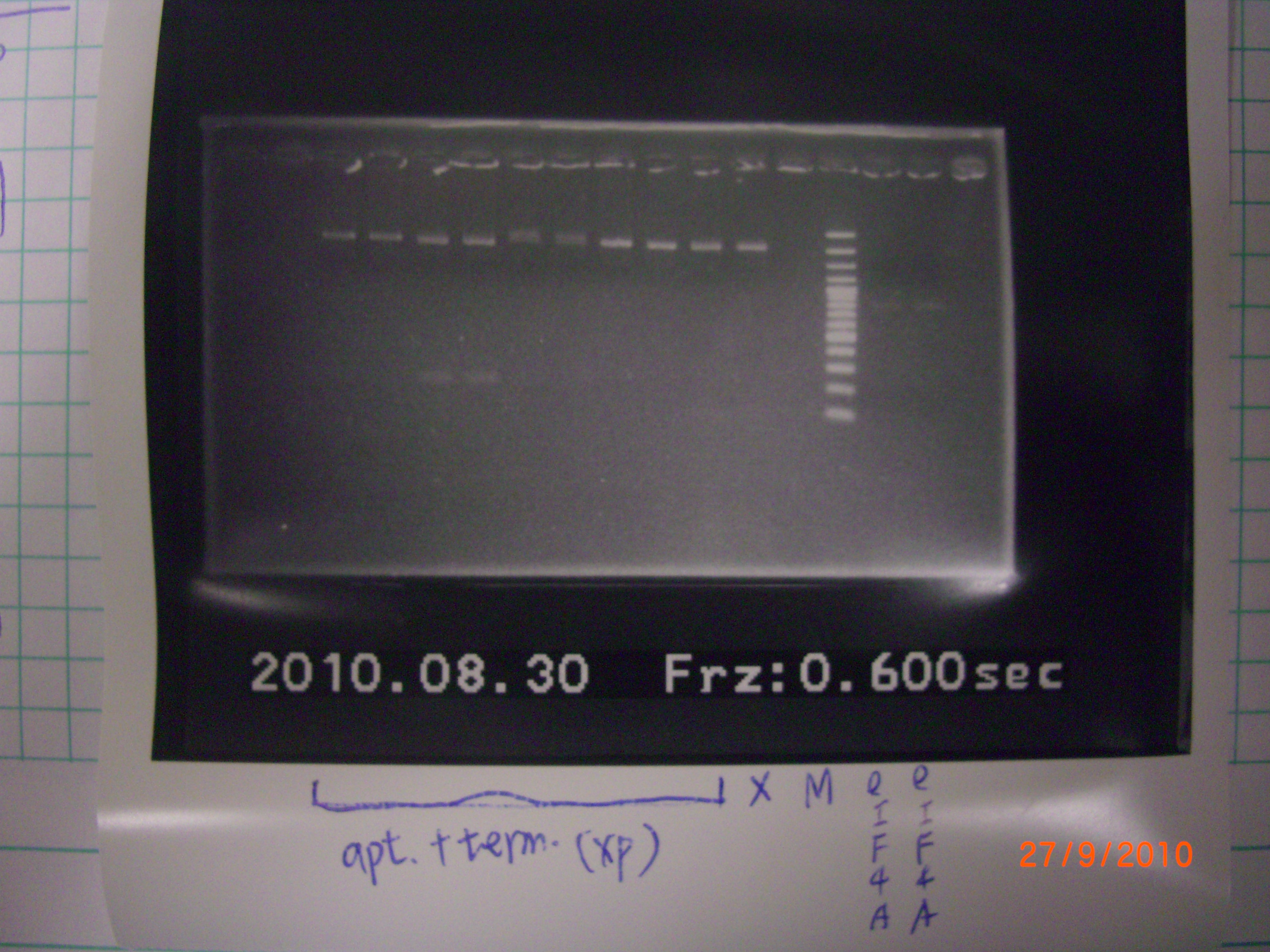 bend1~10 → apt.+ter.(XP) // maker // eIF4A
- ligation of apt.+ter(XP)+(RBS(SP)
- transformation
2010.08.31
- 3 in 1 of apt+term+RBS
- colony PCR of apt+term+RBS
| Total: | 50μl | X2μl | | | FP | RP | Template
|
| Template | 1μl | | | negative | VF2 | VR |
|
| FP | 1μl | 5μl | | positive | VF2 | VR | complete GFP
|
| RP | 1μl | 5μl |
|
| dNTP | 2μl | 10μl |
|
| 10XBuff. | 5μl | 25μl |
|
| taq | 0.25μl | 1.25μl |
|
| ddH2O | 39.75μl | 198.75μl |
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 40s
| 30 cycles
|
| 72
| 300s
|
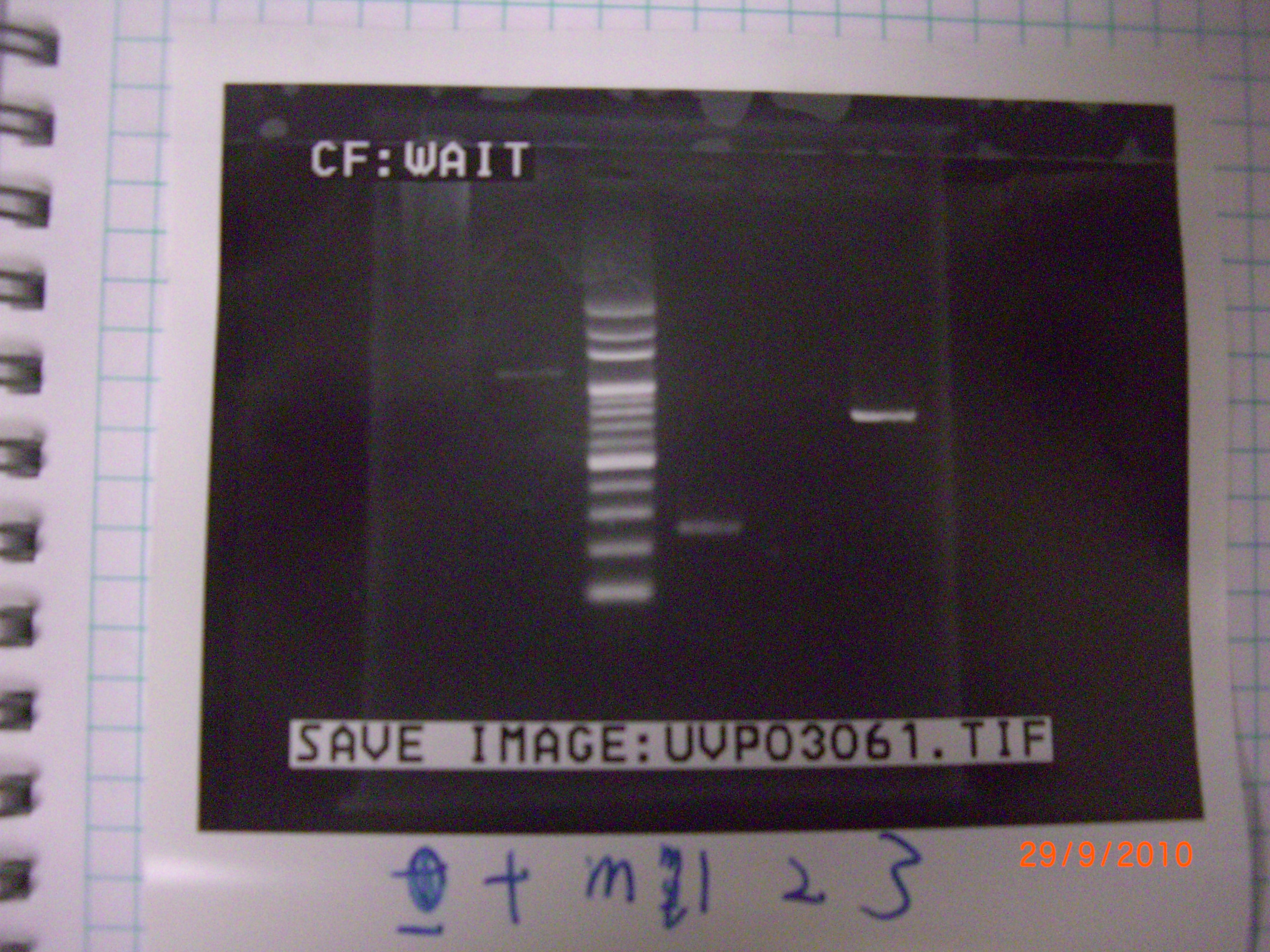
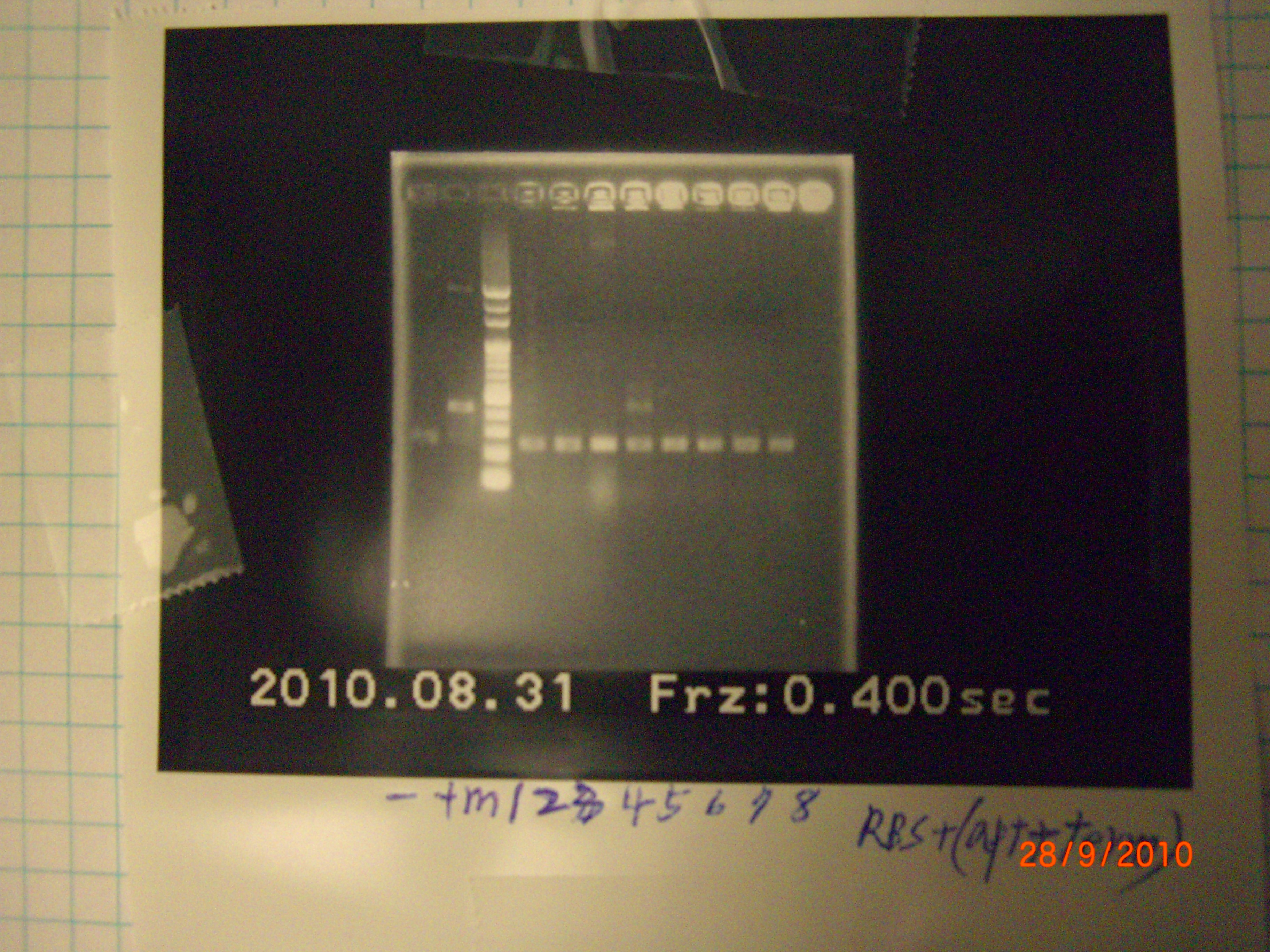 tube 4 ligase successfully. - colony PCR(for test) of eIF4A 1,2,3(mutant part 1.2.3) >>>> 55'C
| Total: | 50μl | X2.5μl | | | FP | RP | Template
|
| Template | 1μl | 5.6μl | | negative | | |
|
| FP | 1μl | | | positive | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A/C212-2
|
| RP | 1μl | | | mutant1 | eIF4A-split-A-FP | eIF4A-mutant-1-RP | eIF4A/C212-2
|
| dNTP | 2μl | 5μl | | mutant2 | eIF4A-mutant-1-FP | eIF4A-mutant-2-RP | eIF4A/C212-2
|
| 10XBuff. | 5μl | 12.5μl | | mutant3 | eIF4A-mutant-2-FP | eIF4A-split-B-RP | eIF4A/C212-2
|
| taq | 0.25μl | 0.625μl |
|
| ddH2O | 39.75μl | 99.375μl |
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 53.6
| 20s
|
| 72
| 80s
| 30 cycles
|
| 72
| 300s
|
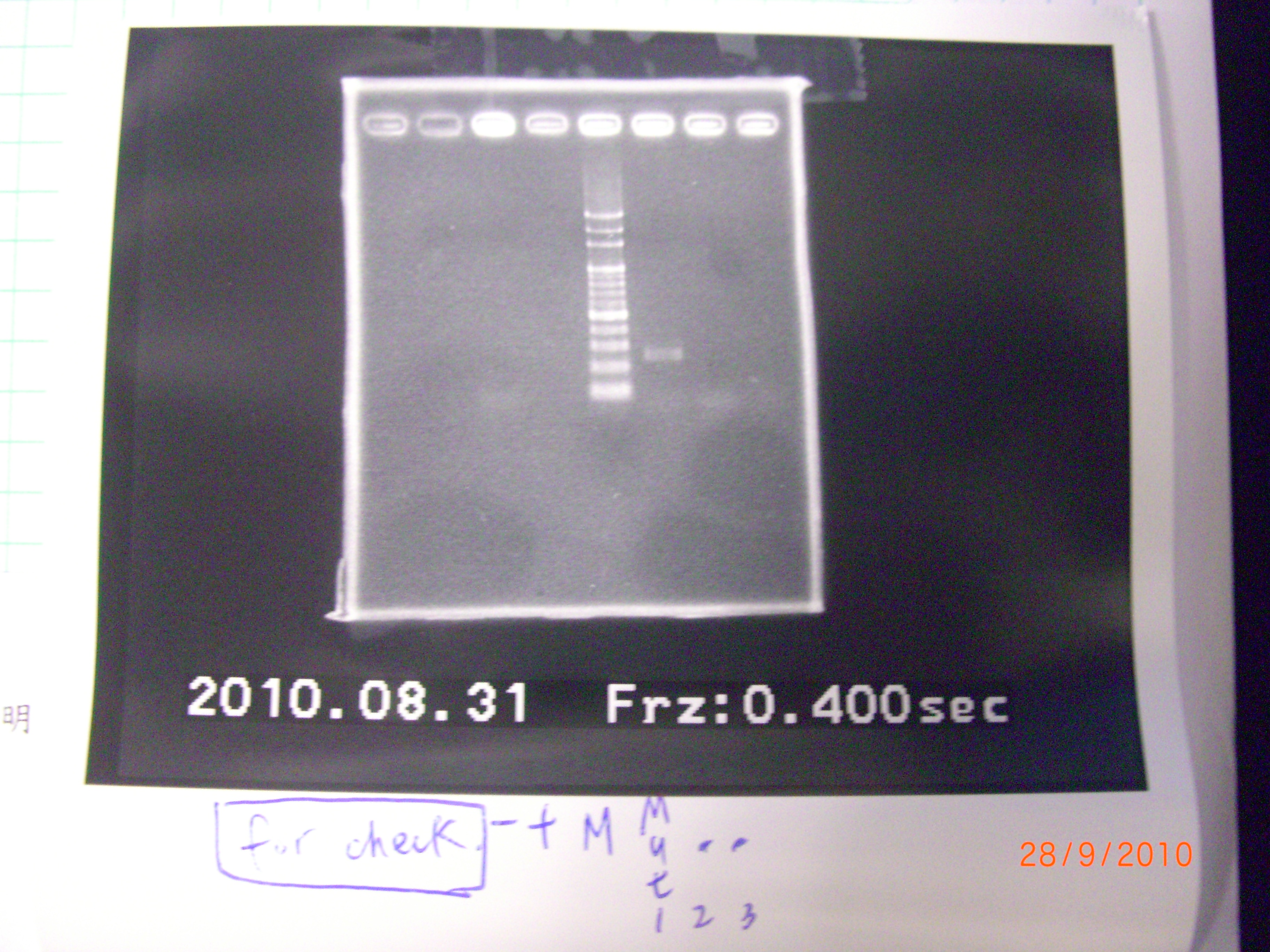 mut.2 and3 didn't have bends, so we PCR test again. - colony PCR(for test) of eIF4A 1,2,3(mutant part 1.2.3)>>>>>>>> for the second time! >>>> 51'C
2010.09.01
- plasimd extraction of apt.+term.+RBS(X2 tubes)
- digestion of apt.+term.+RBS(XP) and use our own pLac(SP) for ligation
- digestion protocol of apt.+term.+RBS(XP)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- run gel >> gel extraction of apt.+term.+RBS(XP)
- purification of apt.+term.+RBS(XP)>> ligation >> transformation(9/1 18:00)
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(apt.+ter+RBS[XP]) | 6.7μl | length:235
|
| DNA2(pLac[SP]) | 1.8μl | length:200
|
- eIF4A PCR test result(yesterday)
| Total: | 50μl | X2μl | | | FP | RP | Template | length
|
| Template | 1μl | | | negative | | | |
|
| FP | 1μl | | | positive | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A/C212-2 | 1216
|
| RP | 1μl | | | mutant1 | eIF4A-split-A-FP | eIF4A-mutant-1-RP | eIF4A/C212-2 | 254
|
| dNTP | 4μl | 8μl | | mutant2 | eIF4A-mutant-1-FP | eIF4A-mutant-2-RP | eIF4A/C212-2 | 191
|
| 10XBuff. | 5μl | 10μl | | mutant3 | eIF4A-mutant-2-FP | eIF4A-split-B-RP | eIF4A/C212-2 | 811
|
| pfu | 1μl | 2μl
|
| ddH2O | 37μl | 74μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 51
| 30s
|
| 72
| 160s
| 35 cycles
|
| 72
| 60s
|
2010.09.02
- 3 in 1 of (apt.+term.+RBS)+(pLac) >> PCR >> run gel for check
| Total | 661
|
| aptamer+scar | 58+8
|
| terminator+scar | 129+8
|
| RBS+scar | 12+8
|
| pLac | 200
|
| VR-VF2 | 238
|
- PCR protocol for test(6tubes)
| Total: | 50μl | X3μl | | FP | RP | Template
|
| Template | 1μl | | negative | VF2 | VR |
|
| FP | 1μl | 3μl | positive | VF2 | VR | complete GFP
|
| RP | 1μl | 3μl
|
| dNTP | 2μl | 6μl
|
| 10XBuff. | 5μl | 15μl
|
| taq | 0.25μl | 0.75μl
|
| ddH2O | 39.75μl | 119.25μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 60s
| 30 cycles
|
| 72
| 300s
|
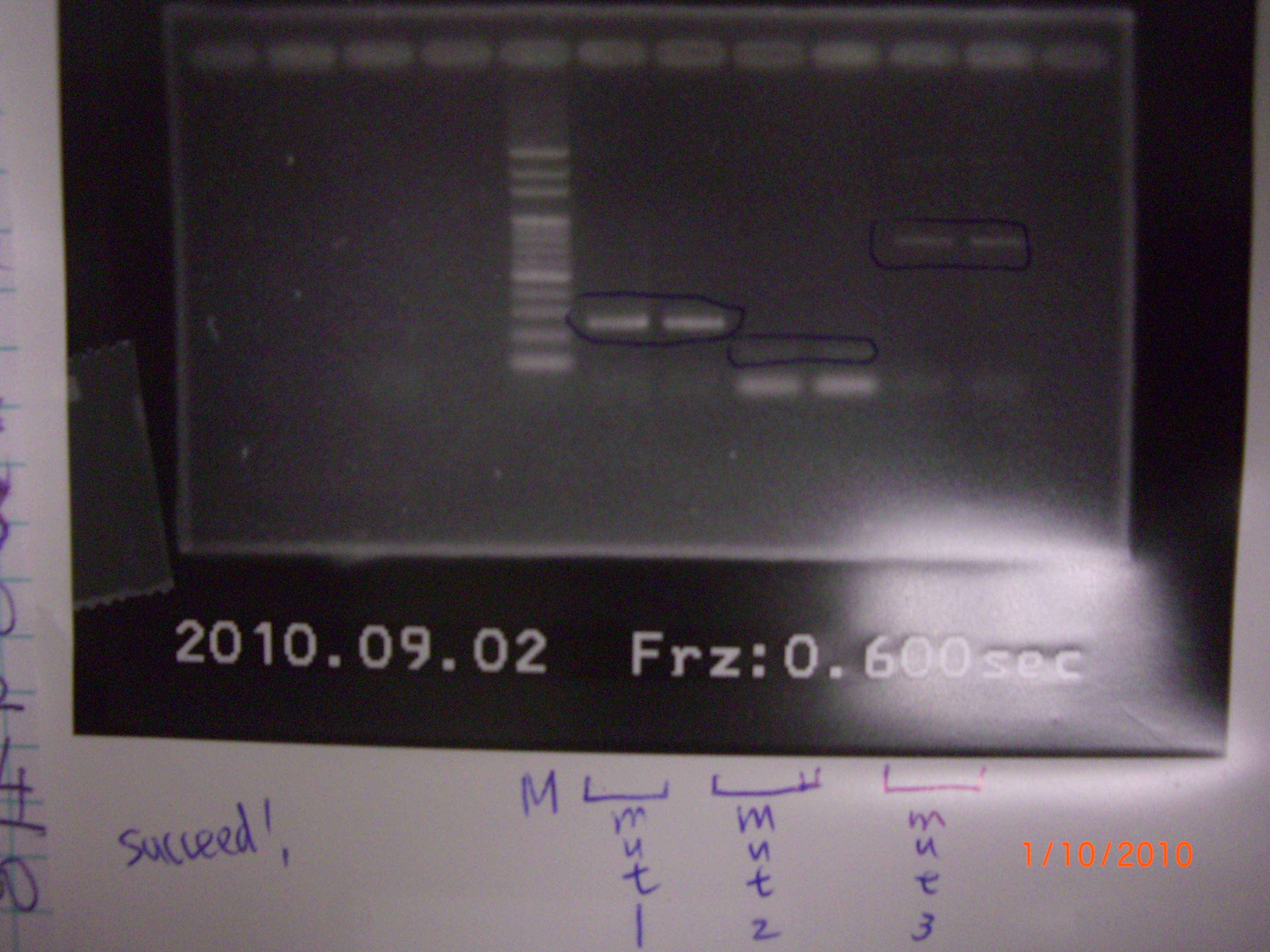
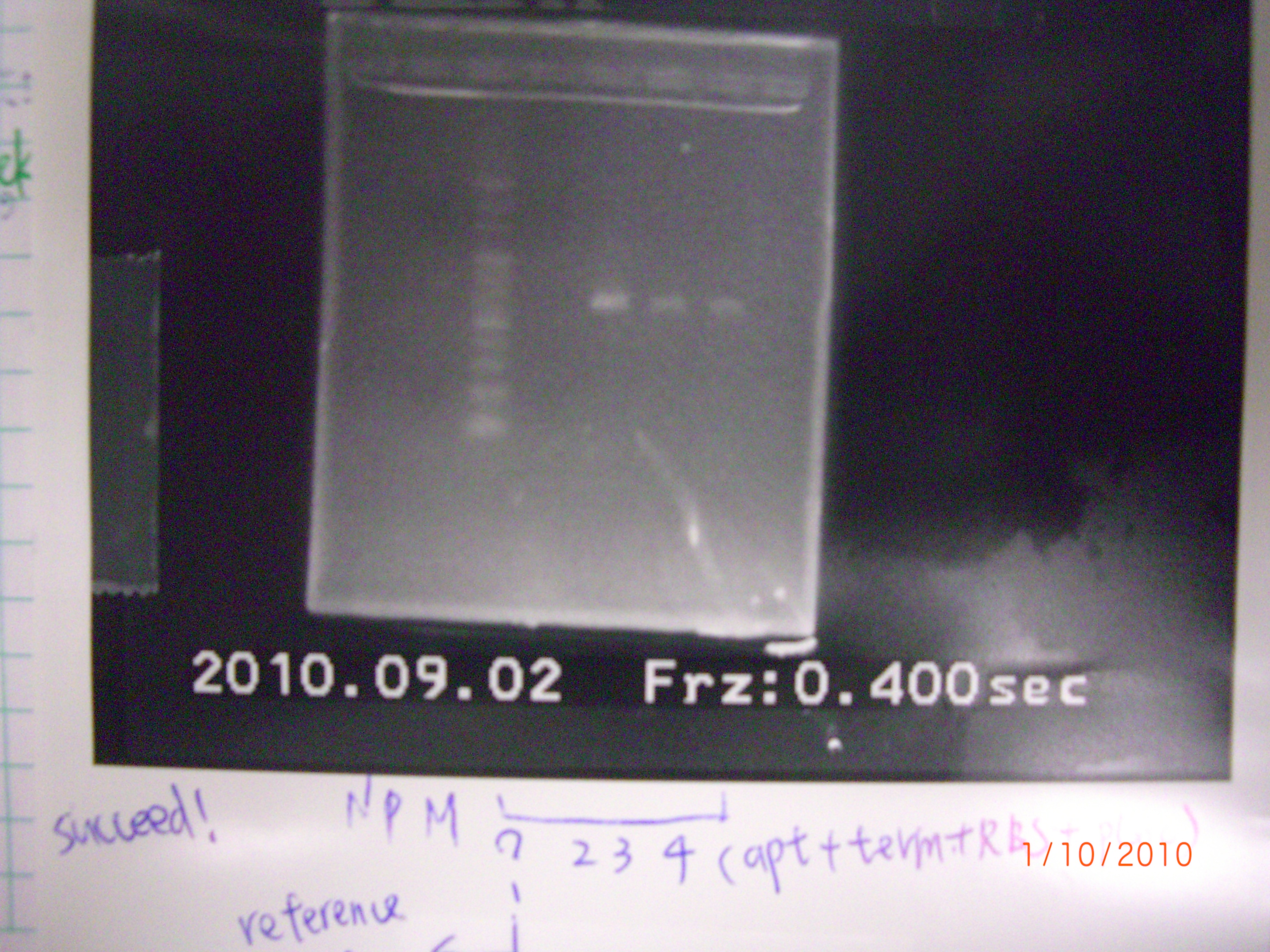 all succeed.(tube1 was taken by SsrA group as negative.= = - run gel of eIF4A mutant1.2.3 >> gel extraction >> purification >> PCR of mutant1+2 and mutant2+3
- run gel result of mutant1.2.3
- PCR protocol for test(4tubes)
| Total: | 50μl | X2μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A/C212-2 | 1216
|
| RP | 1μl | | mutant1+2 | eIF4A-split-A-FP | eIF4A-mutant-2-RP | eIF4A/C212-2 | 445-8
|
| dNTP | 4μl | 8μl | mutant2+3 | eIF4A-mutant-1-FP | eIF4A-split-B-RP | eIF4A/C212-2 | 1002-8
|
| 10XBuff. | 5μl | 10μl
|
| pfu | 1μl | 2μl
|
| ddH2O | 37μl | 74μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 51
| 30s
|
| 72
| 160s
| 35 cycles
|
| 72
| 600s
|
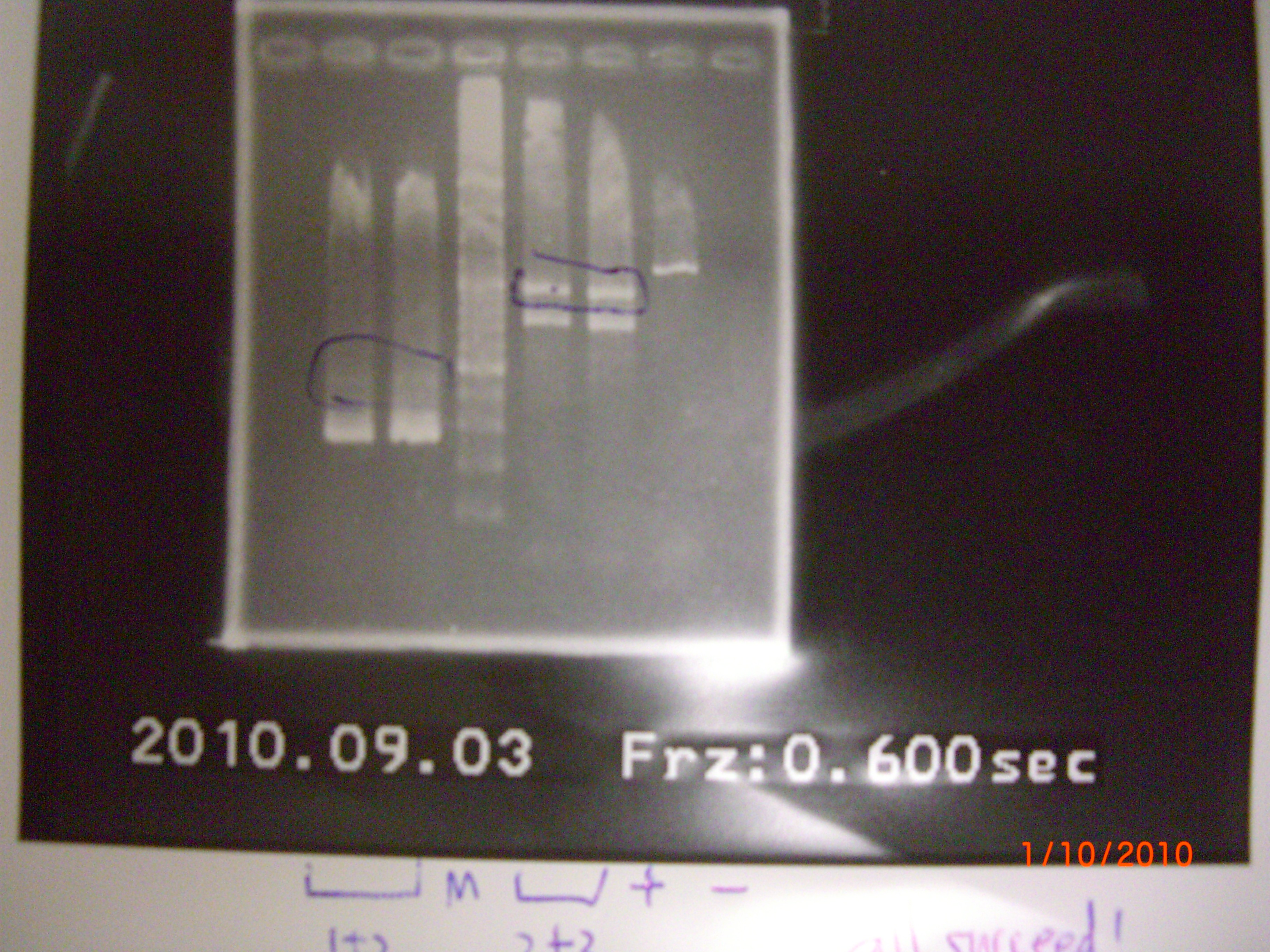
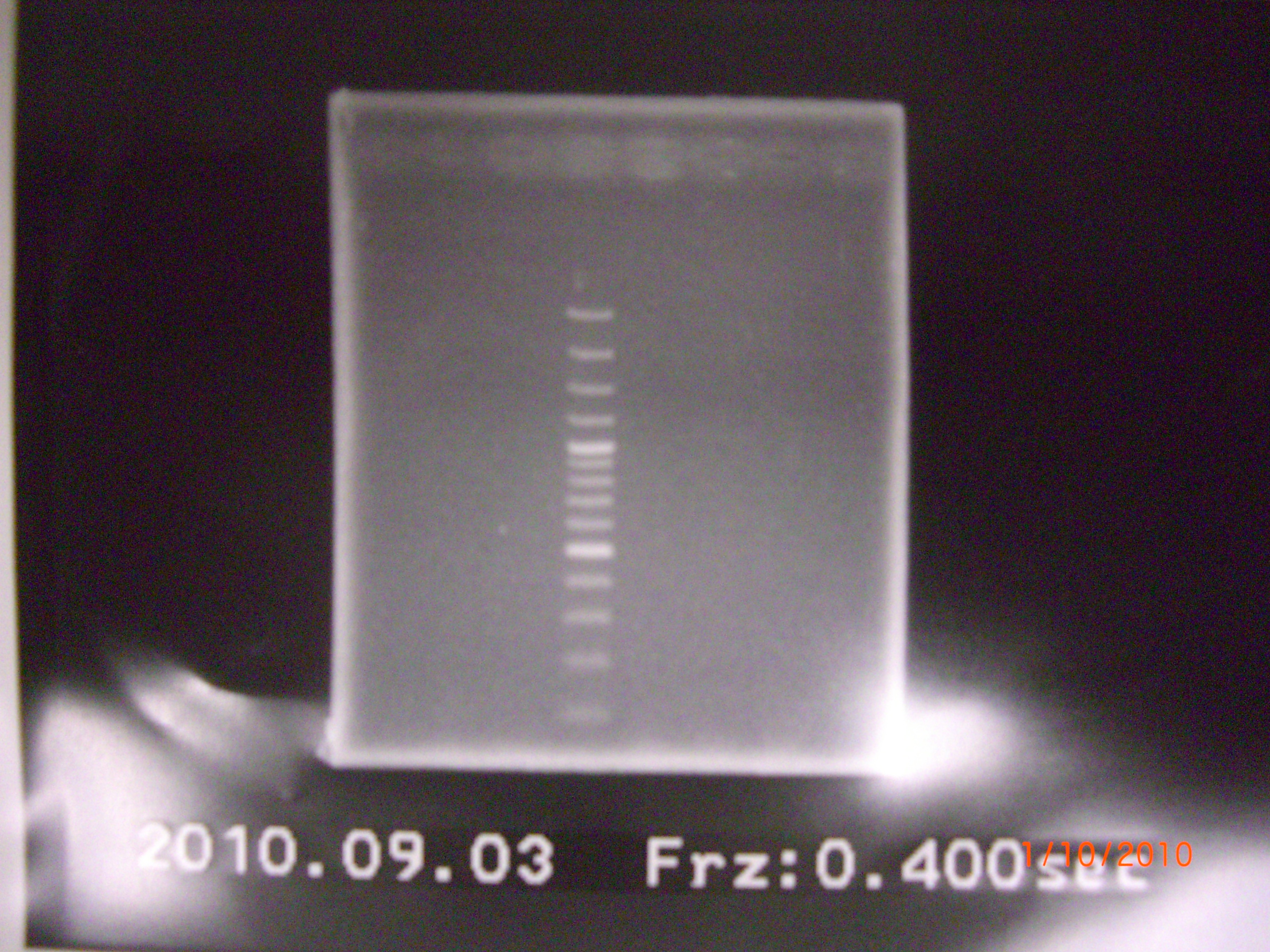 all failed.So we do again at 9/3. 2010.09.03
- plasimd extraction of aptamer+terminator+RBS+pLac, and the aptamer part have been done!!! (can use any other promotor)
- PCR of eIF4A mutant1+2, mutant2+3 again >> run gel >> gel extraction >> purification
- protocol as yesterday.
- result
- PCR of eIF4A mutant(1+2)+3, mutant1+(2+3)
| Total: | 50μl | X2μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A/C212-2 | 1216
|
| RP | 1μl | | mutant(1+2)+3 | eIF4A-split-A-FP | eIF4A-split-B-RP | mutant(1+2) | 1216
|
| dNTP | 4μl | 8μl | mutant1+(2+3) | eIF4A-split-A-FP | eIF4A-split-B-RP | mutant(2+3) | 1216
|
| 10XBuff. | 5μl | 10μl
|
| pfu | 1μl | 2μl
|
| ddH2O | 37μl | 74μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 51
| 30s
|
| 72
| 160s
| 35 cycles
|
| 72
| 600s
|
2010.09.04
2010.09.05
2010.09.06
2010.09.07
2010.09.08
- 3 in 1 of pSB1C3+aptamer/+apt+term/+RBS+apt+term/+pLac+RBS+apt+term
- colony PCR (12 tubes)
- pSB1C3+RBS+apt+term and pSB1C3+pLac+RBS+apt+term need to be transformed again( Because there are no coloines on plate.)
2010.09.09
- elute dried DNA(pGEX-eIF4A) from kind Pro.C.Proud(use 60ul TE buffer and put at 4'C overnight)
- plasmid extraction of pSB1C3+aptamer and pSB1C3+pLac
- run gel of (1) pSB1C3+aptamer+terminator (2) pSB1C3+pLac (3) posotive (4) negative
- result of the above: all succeed!!So, pSB1C3+pLac can be put on our 2010 biobrick!(LB tube of pSB1C3+aptamer have nothing.)
- ligation of (pLac+RBS+aptamer+terminator)+pSB1C3 >> transformation
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1C3) | 2μl
|
| DNA2(pLac+RBS+aptamer+terminator) | 3μl
|
| ddH2O | 3.5μl
|
2010.09.10
- 3 in 1 of pSB1C3+pLac+RBS+apt+term and pSB1C3+RBS+apt+term(because LB tube did not be put for cultivating><)
- ligation of (pSB1C3)+(apt+term) >> transformation
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1C3) | 1μl
|
| DNA2(pLac+RBS+aptamer+terminator) | 1.5μl
|
| ddH2O | 6μl
|
- PCR colony of (1.2) pSB1C3+pLac+RBS+apt+term (3) pSB1C3+aptamer (4) pSB1C3+RBS+apt+term
- PCR protocol for test(6tubes)
| Total: | 50μl | X3μl | | FP | RP | Template
|
| Template | 1μl | | negative | VF2 | VR |
|
| FP | 1μl | 3μl | positive | VF2 | VR | pLac+RBS+apt+term
|
| RP | 1μl | 3μl
|
| dNTP | 2μl | 6μl
|
| 10XBuff. | 5μl | 15μl
|
| taq | 0.25μl | 0.75μl
|
| ddH2O | 39.75μl | 119.25μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 60s
| 30 cycles
|
| 72
| 300s
|
2010.09.11
- plasmid extraction of pSB1C3+pLac+RBS+aptamer+terminator and pSB1C3+RBS+aptamer+terminator succeed! Have two new 2010 biobricks!
- The PCR of yesterday failed. So, do it again. And the result of the PCR today sill failed.(no bends><), we still figure what the problem is.
- The plate of pSB1C3+aptamer+terminator have no colonies, so ligate again.
- digestion of pSB1C3(XP)
| Total | 20μl
|
| DNA | 10μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 6μl
|
- ligation of pSB1C3+aptamer+terminator >> transformation
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1C3) | 3.5μl
|
| DNA2(aptamer+terminator) | 1.5μl
|
| ddH2O | 3.5μl
|
2010.09.12
- colony PCR of complete eIF4A
| Total: | 50μl | X1.5μl | | FP | RP | Template
|
| Template | 1μl | | negative | VF2 | VR |
|
| FP | 1μl | 1.5μl | positive | VF2 | VR | complete GFP
|
| RP | 1μl | 1.5μl
|
| dNTP | 2μl | 3μl
|
| 10XBuff. | 5μl | 7.5μl
|
| taq | 0.25μl | 0.375μl
|
| ddH2O | 39.75μl | 596.25μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 51
| 20s
|
| 72
| 80s
| 30 cycles
|
| 72
| 300s
|
2010.09.13
- transform (pGEX-eIF4A)(because the concentration of template from C.Proud is too low)
- PCR(3 tubes) Test
| Total: | 50μl | X1.5μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive | VF2 | VR | GFP |
|
| RP | 1μl | | eIF4A(0.3+water0.2) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| dNTP | 4μl | 3μl
|
| 10XBuff. | 5μl | 7.5μl
|
| pfu | 1μl | 0.375μl
|
| ddH2O | 37μl | 59.625μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 80s
| 30 cycles
|
| 72
| 300s
|
2010.9.14
| Total: | 50μl | X3μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | 3μl | positive(eIF4A) | VF2 | VR | GFP |
|
| RP | 1μl | 3μl | pGEX-eIF4A(1) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| dNTP | 2μl | 6μl | pGEX-eIF4A(2) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| 10XBuff. | 5μl | 15μl | pGEX-eIF4A(3) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| taq | 0.25μl | 0.75μl | pGEX-eIF4A(4) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| ddH2O | 39.75μl | 119.25μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 80s
| 30 cycles
|
| 72
| 300s
|
2010.9.15
- real PCR(5tubes) of split eIF4A mutI, mutII, mutIII
| Total: | 50μl | X2.5μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | 2.5μl | positive(eIF4A) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| RP | 1μl | 2.5μl | mutI(1) | eIF4A-split-A-FP | eIF4A-mutI-RP | eIF4A | 258
|
| dNTP | 2μl | 10μl | mutII(2) | eIF4A-mutI-FP | eIF4A-mutII-RP | eIF4A | 191
|
| 10XBuff. | 5μl | 12.5μl | mutIII(3) | eIF4A-mutII-FP | eIF4A-split-B-RP | eIF4A | 811
|
| MgSO4 | 4μl | 10μl |
|
| KOD | 1μl | 2.5μl |
|
| ddH2O | 39.75μl | 82.5μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 160s
| 35 cycles
|
| 72
| 600s
|
| Total: | 50μl | X2μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | 2μl | positive(eIF4A) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| RP | 1μl | 2μl | mutI+mutII | eIF4A-split-A-FP | eIF4A-mutII-RP | mutI+mutII | 424
|
| dNTP | 2μl | 4μl | mutII+mutIII | eIF4A-mutI-FP | eIF4A-split-B-RP | mutII+mutIII | 983
|
| 10XBuff. | 5μl | 10μl | mutI+mutII+mutIII | eIF4A-split-A-FP | eIF4A-split-B-RP | mutI+mutII+mutIII | 1216
|
| pfu | 0.25μl | 0.5μl |
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 160s
| 35 cycles
|
| 72
| 600s
|
2010.9.16
- run gel→gel extraction
- real PCR
- real PCR protocol
| Total: | 50μl | X2μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | 2μl | positive(eIF4A) | eIF4A-split-A-FP | eIF4A-split-B-RP | eIF4A | 1216
|
| RP | 1μl | 2μl | mut(I+II)+III | eIF4A-split-A-FP | eIF4A-split-B-RP | mutI+mut(II+III) | 1216
|
| dNTP | 2μl | 4μl | mutI+(II+III) | eIF4A-split-A-FP | eIF4A-split-B-RP | mutI+mutII+mutIII | 1216
|
| 10XBuff. | 5μl | 10μl | mut(I+II+III) | eIF4A-split-A-FP | eIF4A-split-B-RP | mutI+mutII+mutIII | 1216
|
| pfu | 0.25μl | 0.5μl |
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 160s
| 35 cycles
|
| 72
| 600s
|
- digestion of eIF4A on pGEX
| Total | 20μl
|
| DNA(pGEX-eIF4A1) | 2μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 14μl
|
- digestion of mut(I+II+III)
| Total | 20μl
|
| DNA(mut(I+II)+III) | 10μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 6μl
|
- ligation: (1)eIF4A on pSB1C3 (2)mut(I+II)+III
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(eIF4A) | 3μl
|
| DNA2(pSB1C3) | 2μl
|
| ddH2O | 3.5μl
|
2010.9.17
| Total: | 50μl | X1.5μl | | FP | RP | Template | length
|
| Template | 1.5μl | | negative | | | |
|
| FP | 1μl | | positive(mut(I+II)+III) | eIF4A-split-A-FP | eIF4A-split-B-RP | mut(I+II)+III | 1216
|
| RP | 1μl | | mut(I+II)+III | eIF4A-FP | eIF4A-split-B-RP | mutI+mut(II+III) | 1216
|
| dNTP | 2μl | 3μl |
|
| 10XBuff. | 5μl | 7.5μl |
|
| pfu | 0.25μl | 0.375μl |
|
| ddH2O | 39.75μl | 59.625μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 80s
| 35 cycles
|
| 72
| 420s
|
2010.9.18
2010.9.20
- purification of eIF4A(I+II)+III
- ligation and transformation of eIF4A(I+II)+III and pSB1C3
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(eIF4A) | 3μl | eIF4A length 1216
|
| DNA2(pSB1C3) | 2μl | pSB1C3 length 316
|
| ddH2O | 3.5μl
|
- PCR of eIF4A-A,eIF4A-B(4tubes)
| Total: | 50μl | X2μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive(eIF4A) | eIF4A-split-A-FP | eIF4A-split-B-RP | c212-2 | 1216
|
| RP | 1μl | | eIF4A-A | eIF4A-split-A-FP | eIF4A-split-A-RP | mut(I+(II+III)) | 1216
|
| dNTP | 2μl | 4μl | eIF4A-B | eIF4A-split-B-FP | eIF4A-split-B-RP | mut(I+(II+III)) | 1216
|
| 10XBuff. | 5μl | 10μl |
|
| pfu | 0.25μl | 0.5μl |
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 80s
| 35 cycles
|
| 72
| 420s
|
2010.9.21
- PCR of GFP-A,GFP-B,RFP-A,RFP-B(6tubes)
| Total: | 50μl | X3μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive(RFP) | VF2 | VR | RFP | 776
|
| RP | 1μl | | GFP-A | GFP-split-A-FP | GFP-split-A-RP | GFP | 517
|
| dNTP | 2μl | 6μl | GFP-B | GFP-split-B-FP | GFP-split-B-RP | GFP | 298
|
| 10XBuff. | 5μl | 15μl | RFP-B | RFP-split-B-FP | RFP-split-B-RP | GFP | 508
|
| pfu | 0.25μl | 0.75μl | RFP-B | RFP-split-B-FP | RFP-split-B-RP | GFP | 268
|
| ddH2O | 39.75μl | 119.25μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 80s
| 35 cycles
|
| 72
| 420s
|
- 3 in 1 of eIF4A(I+II)+III and pSB1C3
- PCR of eGFP(A)+eIF4A(A),eGFP(B)+eIF4A(B)
| Total: | 50μl | X2μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive | VF2 | VR | eIF4A(eIF4A on pGEX) | 1216
|
| RP | 1μl | | system GA | GFP-split-A-FP | eIF4A-split-A-RP | GFP | 1195
|
| dNTP | 2μl | 4μl | system GB | GFP-split-B-FP | eIF4A-split-B-RP | GFP | 847
|
| 10XBuff. | 5μl | 10μl | system RA | RFP-split-A-FP | eIF4A-split-A-RP | GFP | 1181
|
| pfu | 0.25μl | 0.5μl | system RB | RFP-split-A-FP | eIF4A-split-A-RP | GFP | 818
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 80s
| 35 cycles
|
| 72
| 420s
|
2010.9.22
- gel extraction of system GA,system GB,system RA,system RB
- digestion of eIF4A on pSB1C3,system GA,system GB, system RA,system RB
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- ligation of system GA,system GB,system RA
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(GA or GB) | 0.5μl
|
| DNA2(pSB1C3) | 4.5μl
|
| ddH2O | 3.5μl
|
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(~) | 1.5μl
|
| DNA2(pSB1C3) | 3.5μl
|
| ddH2O | 3.5μl
|
2010.9.23
- digstion of pSB1C3+pLac(X,P)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- gel extraction
- ligation of system GA,system GB,system RA,system RB,
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(~) | 2.5μl
|
| DNA2(pSB1C3(X,P)) | 5μl
|
| ddH2O | 1μl
|
2010.9.24
- 3 in 1 of system GA, system GB, system RA, system RB
- colony PCR of system GA, system GB, system RA, system RB
| Total: | 50μl | X5μl | | FP | RP | Template | length
|
| Template | 1μl | | negative | | | |
|
| FP | 1μl | | positive(eIF4A on pGEX) | eIF4A-split-A-Fp | eIF4A-split-B-Rp | eIF4A on pGEX | 1216
|
| RP | 1μl | | system GA on pSB1C3 | GFP-split-A-FP | GFP-split-A-RP | GFP | 1195
|
| dNTP | 2μl | 10μl | system GB on pSB1C3 | GFP-split-B-FP | GFP-split-B-RP | GFP | 847
|
| 10XBuff. | 5μl | 25μl | system RA on pSB1C3 | RFP-split-B-FP | RFP-split-B-RP | GFP | 1181
|
| pfu | 0.25μl | 1.25μl | system RB on pSB1C3 | RFP-split-B-FP | RFP-split-B-RP | GFP | 818
|
| ddH2O | 39.75μl | 198.75μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 80s
| 35 cycles
|
| 72
| 300s
|
2010.09.25
(1)GFP-eIF4A-A-1
(2)GFP-eIF4A-A-2(failed)
(3)GFP-eIF4A-B-1
(4)GFP-eIF4A-B-2(failed)
(5)RFP-eIF4A-A-1
(6)RFP-eIF4A-A-2
(7)RFP-eIF4A-B-1(failed)
(8)RFP-eIF4A-B-2
- digestion of GA(XP),GB(XP),RA-1(XP),RA-2(XP),pGEX-e(P),pGEX-e(X)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- ligation of GA,GB,RA,RB
- ligation protocol of GA,GB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(GA/GB)) | 5μl
|
| ddH2O | 1.5μl
|
- ligation protocol of RA,RB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(RA/RB)) | 1μl
|
| ddH2O | 5.5μl
|
- digestion of pSB1A2(XP), pSB1C3(XP)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
2010.09.26
- colony PCR of GA(X2),GB(X2),RA(X2),RB(X2),on pSB1A2
| Total: | 50μl | X6μl | length
|
| Template | 1μl | | negative
|
| FP(VF2) | 1μl | | positive(GFP2) | 720+238
|
| RP(VR) | 1μl | | GFP-eIF4A-A-1,2 | 1387
|
| dNTP | 2μl | 12μl | GFP-eIF4A-B-1,2 | 1042
|
| 10XBuff. | 5μl | 30μl | RFP-eIF4A-A-1,2 | 1387
|
| tag | 0.25μl | 1.5μl | RFP-eIF4A-B-1,2 | 1012
|
| ddH2O | 39.75μl | 238.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
- digestion and gel extration of pSB1C3(XP), pSB1A2(XP)
2010.09.27
- plasmid extraction of GFP-e-A-2,GFP-e-B-2,RFP-e-A-2,RFP-e-B-2
- digestion of GFP-e-A-2,GFP-e-B-2,RFP-e-A-2,RFP-e-B-2
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- run gel and check.....failed!
- ligation of GA,GB,RA,RB
- ligation protocol of GA,GB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(GA/GB)) | 5μl
|
| ddH2O | 1.5μl
|
- ligation protocol of RA,RB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(RA/RB)) | 1μl
|
| ddH2O | 5.5μl
|
2010.09.28
- 3 in 1 of colony PCR of GA,GB,RA,RB
| Total: | 50μl | X5μl | length
|
| Template | 1μl | | negative
|
| FP(VF2) | 1μl | | positive(GFP2) | 720+238
|
| RP(VR) | 1μl | | GFP-eIF4A-A-1,2 | 1387
|
| dNTP | 2μl | 10μl | GFP-eIF4A-B-1,2 | 1042
|
| 10XBuff. | 5μl | 25μl | RFP-eIF4A-A-1,2 | 1387
|
| tag | 0.25μl | 1.25μl | RFP-eIF4A-B-1,2 | 1012
|
| ddH2O | 39.75μl | 198.75μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
2010.09.29
- plasmid extractio of GA(1),GB(2),RA(1),RB(2)
- digestion of GA(1),GB(2),RA(1),RB(2)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
2010.09.30
- ligation of GA,GB,RA,RB on pSB1A2
- ligation protocol of GA,GB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(GA/GB)) | 5μl
|
| ddH2O | 1.5μl
|
- ligation protocol of RA,RB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(RA/RB)) | 1μl
|
| ddH2O | 5.5μl
|
2010.10.01
- 3in1 of GA(2),GB(2),RA(1),J13002(2) (use liquid to replace for the LB buffer)
| Total: | 50μl | X4.5μl |
|
| Template | 1μl | | negative |
|
| FP | 1μl | | positive(GFP2) |
|
| RP | 1μl | |
|
| dNTP | 2μl | 9μl |
|
| 10XBuff. | 5μl | 22.5μl |
|
| tag | 0.25μl | 1.125μl |
|
| ddH2O | 39.75μl | 178.875μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 80s
| 35 cycles
|
| 72
| 300s
|
- digestion GeA,GeB,ReA,ReB
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| Total: | 50μl | X4μl | | FP | RP | Template | length
|
| Template | 1μl | | negative |
|
| FP | 1μl | |
|
| RP | 1μl | | system GA | GFP-split-A-FP | eIF4A-split-A-RP
|
| dNTP | 2μl | 8μl | system GB | GFP-split-B-FP | eIF4A-split-B-RP
|
| 10XBuff. | 5μl | 20μl | system RA | RFP-split-A-FP | eIF4A-split-A-RP
|
| pfu | 0.25μl | 1μl | system RB | RFP-split-B-FP | eIF4A-split-B-RP
|
| ddH2O | 39.75μl | 159μl
|
| PCR Protocol |
|
| 94
| 120s
|
| 94
| 30s
|
| 55
| 30s
|
| 72
| 80s
| 35 cycles
|
| 72
| 420s
|
2010.10.02
- plasmid extraction of GA, GB, RA, J13002
- digestion(XP) to check length
- purify the PCR yesterday and digest(DNA:16)
- gel extraciton
- GA.RB digest again.
- RUSULT:
- THe result of digestion from plasmid extraction is wrong.
- The reusult of PCR is right
2010.10.03
- colony PCR of GeA, GeB, ReA, ReB on J13002
| Total: | 50μl | X10μl
|
| Template | 1μl |
|
| FP | 1μl | | positive(GFP2)
|
| RP | 1μl | | system GA on J13002
|
| dNTP | 2μl | 20μl | system GB on J13002
|
| 10XBuff. | 5μl | 50μl | system RA on J13002
|
| pfu | 0.25μl | 2.5μl | system RB on J13002
|
| ddH2O | 39.75μl | 397.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
- ligation of aptamer(XP)+pLac(SP)
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pLac) | 1μl
|
| DNA2(aptamer) | 3μl
|
| ddH2O | 4.5μl
|
| Total | 20μl
|
| DNA | 10μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (S) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 6μl
|
2010.10.04
- purification of J13002(SP)
- 3in1 of aptamer(XP)+pLAC(SP)
| Total: | 50μl | X2μl |
|
| Template | 1μl | | negative |
|
| FP | 1μl | | positive(GFP2) |
|
| RP | 1μl | |
|
| dNTP | 2μl | 4μl |
|
| 10XBuff. | 5μl | 10μl |
|
| tag | 0.25μl | 0.5μl |
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 40s
| 30 cycles
|
| 72
| 300s
|
- ligation of J13002(SP)+GA,GB,RA,RB(XP)
- ligation protocol of GA,GB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(J13002) | 4μl
|
| DNA2(~) | 1μl
|
| ddH2O | 3.5μl
|
- ligation protocol of RA,RB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(J13002) | 4.5μl
|
| DNA2(~) | 0.5μl
|
| ddH2O | 3.5μl
|
2010.10.06
- 3in1 of pTet+RBS+GA,GB,RA,RB
| Total: | 50μl | X6μl |
|
| Template | 1μl |
|
| FP | 1μl |
|
| RP | 1μl |
|
| dNTP | 2μl | 12μl
|
| 10XBuff. | 5μl | 30μl
|
| pfu | 0.25μl | 1.5μl
|
| ddH2O | 39.75μl | 238.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
- plasmid extraction of aptamer(XP)+pLac(SP)]
- run gel of pTet+RBS+GA(X5),GB(X4),RA(X4),RB(X4)
- cultivate LB: GA tube5, GB tube3, RA tube1, RA tube2
2010.10.07
- plasmid extraction of pTet+RBS+GA,GB,RA1,RA2
- digestion of pTet+RBS+GA,GB,RA1,RA2
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- digestion of J13002(SP) > purification > ligation of J13002(SP)+RBS(XP)
2010.10.08
- purification of pLac+apt(SP)
- digestion of pSB1C3(XP),pLac+apt(XP)
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- run gel of pSB1C3(XP),pLac+apt(XP),terminator(XP) then gel extraction.
- PCR of J13002+GB,J13002+RB1,J13002+RB2
| Total: | 50μl | X2μl
|
| Template | 1μl
|
| FP | 1μl |
|
| RP | 1μl |
|
| dNTP | 2μl | 4μl
|
| 10XBuff. | 5μl | 10μl
|
| tag | 0.25μl | 0.5μl
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
- 3in1 of J13002+GB,J13002+RB1,J13002+RB2
2010.10.09
- PCR of J13002+GB,J13002+RB1,J13002+RB2
| Total: | 50μl | X2μl
|
| Template | 1μl
|
| FP | 1μl |
|
| RP | 1μl |
|
| dNTP | 2μl | 4μl
|
| 10XBuff. | 5μl | 10μl
|
| tag | 0.25μl | 0.5μl
|
| ddH2O | 39.75μl | 79.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
- plasmid extraction of J13002+GB,J13002+RB1,J13002+RB2
- digestion of J13002+GB,J13002+RB1,J13002+RB2
| Total | 20μl
|
| DNA | 16μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (X) | 1μl
|
| Enzyme 2 (P) | 1μl
|
- run gel to check >> all failed.
- purification of
(1)pLac+aptamer(XP)
(2)terminator(XP)
(3)pSB1C3(XP)
(1)eIF4A+pSB1C3
(2)pLac+aptamer(SP)+terminator(XP)
(3)J13002(SP)+GA/GB/RA/RB
- ligation protocol of GA,GB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(GA/GB)) | 5μl
|
| ddH2O | 1.5μl
|
- ligation protocol of RA,RB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1A2) | 2μl
|
| DNA2(GA/GB)) | 5μl
|
| ddH2O | 1.5μl
|
2010.10.10
- colony PCR of pSB1C3+eIF4A,pSB1C3+pLac+aptamer,J13002+ReB,J13002+GeB
| Total: | 50μl | X2.5μl
|
| Template | 1μl
|
| FP(VF2) | 1μl | 2.5μl
|
| RP(VR) | 1μl | 2.5μl
|
| dNTP | 2μl | 5μl
|
| 10XBuff. | 5μl | 12.5μl
|
| tag | 0.25μl | 0.625μl
|
| ddH2O | 39.75μl | 99.375μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
| Total | 20μl
|
| DNA | 8μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (S) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 8μl
|
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1C3) | 2μl
|
| DNA2(eIF4A) | 3μl
|
| ddH2O | 3.5μl
|
- ligation of pSB1C3+pLac+aptamer,J13002+GeA,J13002+GeB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(pSB1C3/J13002) | 1.5μl
|
| DNA2(pLac+aptamer/GeA,GeB) | 3.5μl
|
| ddH2O | 3.5μl
|
- ligation ofJ13002+ReA,J13002+ReB
| Total | 10μl
|
| buffer | 1μl
|
| ligase | 0.5μl
|
| DNA1(J13002) | 2μl
|
| DNA2(ReA/ReB) | 3μl
|
| ddH2O | 3.5μl
|
2010.10.11
- 3in1 of pSB1C3+GeA,pSB1C3+GeB,pSB1C3+ReA,pSB1C3+ReB,pSB1C3+pLac+aptamer
| Total: | 50μl | X10μl
|
| Template | 1μl
|
| FP | 1μl |
|
| RP | 1μl |
|
| dNTP | 2μl | 20μl
|
| 10XBuff. | 5μl | 50μl
|
| tag | 0.25μl | 2.5μl
|
| ddH2O | 39.75μl | 397.5μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
2010.10.12
- digstion of J13002+GeA,J13002+ReA(x2),J13002+ReB(x2),pSB1C3+pLac+aptamer(x2)
| Total | 20μl
|
| DNA | 8μl
|
| Digestion buffer | 2μl
|
| Enzyme 1 (S) | 1μl
|
| Enzyme 2 (P) | 1μl
|
| ddH2O | 8μl
|
2010.10.13
2010.10.14
2010.10.15
2010.10.16
2010.10.17
2010.10.18
2010.10.19
2010.10.20
2010.10.21
2010.10.22
2010.10.23
2010.10.24
2010.10.25
2010.10.26
2010.10.27
2010.10.28
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 "
"