Team:HokkaidoU Japan/Notebook/August25
From 2010.igem.org
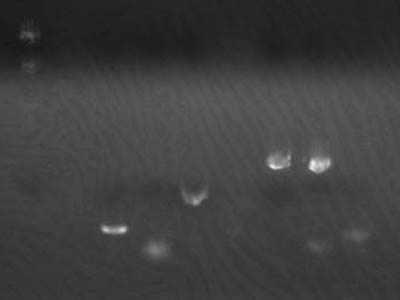
Transformation Results
Colony Check
| Plate | Colonies |
| M 34 | no |
| M 12 | yes |
| I 34 | no |
| I 34 | yes |
| 1-3A 34 | yes |
| 1-3A 12 | yes |
- 1-3A had colonies in lated on mediums containing 34 ug/uL of chloramphenicol
- So the medium and antibiotic consentration seems ok,
- Medium on which M 12 was plated was inoculated with chloramphenicol after solidification so there were some irregularities and colonies on the fringe
- Colonies of 1-3A and 12 were obviously different
→ So we suspected issues in Ligation and DNA concentration bused for transformation
Estimation of 1-3A Consentration
- Added 1 uL of 1-3A into 30 uL of competent cells
- Transformation was successful
- Electrophoresed 1 uL of 1-3A,calculated amount of transformed DNA
→Estimated concentration 2 ng/uL
Ethanol precipitation of pSB1C3 and Ligation
Ethanol precipitation
- Added 8.2 uL of sodium acetate [3 M]
- Added 205 uL of Ethanol [100%] and mixed
- Centrifuge at 15,00rpm, 4C for 10 min
- Discarded supernatant and added 500 uL of Ethanol [70%], mixed
- Centrifuge at 15,00rpm, 4C for 5 min
- Discarded supernatant
- Dried via vacuum desiccator
- Added 8.2 uL of ADW
- Concentration increased 10 fold
Ligation
- Mixed 8.2 uL of DNA solution with the same volume of Ligation Solution
- Added extra 1 uL of T4 ligase
- Incubated at 16C for 30 min
Tomorrow will do aditional Electrophoresis gel extraction and transformation
Digestion of PCR products amplified using digestion visualization primers
Calculated the concentration of DNA amplified yesterday
| RBS | 27 ng/uL | 312 bp |
| GFP | 54 ng/uL | 1020 bp |
| dT | 49 ng/uL | 429 bp |
Digestion Reaction
| Reagent | Amount |
|---|---|
| 10x M buffer | 1 uL |
| DW | 4.5 |
| DNA | 2.5 |
| BSA | 1 |
| EcoR I | 0.5 |
| Pst I | 0.5 |
| Total | 10 uL |
→Incubated at 37C for 60 min
→Confirmed how awesome new primers are "
"